Abstract
Porous carbons were prepared through carbonization of poly(ethylene terephthalate) contained in mixtures with either light basic magnesium carbonate, or magnesium carbonate, or magnesium hydroxide, or magnesium oxide. Pore structure of the obtained carbon materials was presented in relation to a kind of magnesium compound and weight ratio of the raw materials used for preparations. Additionally, an influence of the preparation temperature on the porosity and the pore creation mechanism are briefly discussed. The carbons obtained were tested as sorbent materials for elimination of model contaminants from water. For that purpose, removal of several pollutants of different molecular weights including phenol, Basic Red 18 dye, and Reactive Red 198 dye is presented and discussed in relation to the porosity of the prepared materials.
Similar content being viewed by others
1 Introduction
One of the major attributes of activated carbon is its ability to remove pollutants residing at low concentrations in fluid effluents. For that reason, adsorption onto this porous material is a popular method to remove contaminants from liquid and gaseous streams [1–5]. Adsorptive properties of activated carbons are strongly dependent on their porous structure [6–8]. In general, adsorption of small molecules proceeds effectively in micropores and bigger species require larger pores to be effectively eliminated from the purified media. Hence, an efficient simultaneous removal of contaminants of various molecular sizes is expected to proceed on adsorbents abundant in both micropores and mesopores.
Activated carbons can be produced according to various procedures. Conventionally used physical activation consists of two steps, i.e. carbonization of a suitable carbon precursor followed by activation in steam or CO2 atmosphere at ca. 900 °C [6, 9–11]. Besides, a chemical processes usually employing chemical activators like ZnCl2 or H3PO4 are also in use [6, 11–15]. Natural precursors like coals or wood are the most frequently used raw materials to produce activated carbons [14–16]. Nevertheless, much attention has been paid to invent alternative methods employing synthetic materials as carbon precursors [17–22]. Additionally, in order to simplify the preparation procedure, one-step processes are often proposed. Inagaki et.al. reported a method to obtain mesoporous carbons by carbonization of polymeric precursors (e.g. poly(vinyl alcohol, poly(ethylene terephthalate)) or pitch contained in a mixture with some magnesium compounds including oxide, citrate, or gluconate, followed by removal of inorganics from the obtained products [20–23]. In this way highly porous carbon materials could be obtained without any activation process. As stated by the authors, both area and size of mesopores in resultant nanoporous carbons were strongly dependent on the size of MgO particles formed from their precursors during preparation process. A successful preparation of nanoporous carbons from polymeric precursor was also described in our previous paper [18]. As distinct from the works by the other teams, thermally unstable basic magnesium carbonate (BMC) was used as MgO precursor and poly(ethylene terephthalate) (PET) served as the carbon source. After simple pyrolysis of the BMC/PET mixtures, MgO newly formed was removed from the product. In this manner nanoporous carbons revealing high contributions of both micro- and mesopores could be obtained. As explained in the work in details, development of mesopores was due to MgO presence. On the other hand, gaseous products (CO2 and H2O) of BMC decomposition reacted with the char formed from PET and thus micropores were created. Hence, obtained porous carbons revealed bimodal pore structure that to some extent could be controlled by the ratio of BMC and PET used for preparations. Taking into account above findings, we have extended the gamut of MgO precursors potentially capable to reveal an analogous effect like the BMC used in the earlier work. The novelty of this work is use of both Mg(OH)2 and MgCO3 as pore creating agents. The compounds were thought to be suitable for that purpose because both of them undergo thermal decomposition to MgO and suitable gases, according to following reaction schemes:
-
\( {\text{Mg}}({\text{OH}})_{2} \to {\text{MgO}} + {\text{H}}_{2} {\text{O}} \) (decomp. below 360 °C [24])
-
\( {\text{MgCO}}_{3} \to {\text{MgO}} + {\text{CO}}_{2} \) (decomp. below 550 °C [25])
Hence, we expected to obtain new porous carbon materials of bimodal character, rich in both micropores and mesopores, and therefore being potentially capable to adsorb contaminants of a wide range of molecular sizes. In order to examine performance of all the prepared materials as prospective adsorbents, adsorption of three different model contaminants from water was studied with respect to the textural parameters of the porous carbons.
Partial gasification of the char formed from PET by water released from the magnesium hydroxide was already confirmed in our previous work [26]. Since no results concerning pyrolysis of MgCO3/PET mixtures were reported, a brief discussion on the pore creation mechanism in this system is also provided.
2 Experimental
2.1 Materials and preparations
In this work four sorts of porous carbons prepared from the PET contained in mixtures with different magnesium compounds were studied. The materials were prepared according to the analogous procedures consisting in carbonization of the carbon precursor in mixtures with either MgO precursor (BMC, or MgCO3, or Mg(OH)2) or MgO itself, followed by removal of the residual inorganics by means of acid-washing. Preparation method for the porous carbon materials obtained from MgO/PET and BMC/PET was already described in details in our earlier work [18]. In order to prepare two other sorts of carbon, Mg(OH)2/PET and MgCO3/PET mixtures were pyrolysed at three temperatures, 550, 700, and 850 °C, under flow of high purity (99.999 %) argon gas. The inorganics included in the obtained products were washed out using excessive amounts of aqueous HCl solution and distilled water. Magnesium carbonate (MgCO3, Sigma-Aldrich, Germany) of analytic grade and reagent grade magnesium hydroxide (Mg(OH)2, 95 %, Fluka (Germany) were used in these cases as MgO precursors. A commercial grade PET purchased from Elana S.A. (Poland) served as the carbon precursor. Analytical grade phenol (Sigma-Aldrich, Germany), commercial grade Reactive Red 198 (CAS No. 145017-98-7) and Basic Red 18 (CAS No.14097-03-1) dyes, both obtained from Boruta-Kolor Sp. z o. o., Poland, were employed as model adsorbates.
2.2 Methods
X-ray diffraction pattern presented in this work was measured with use of Philips X’Pert PRO diffractometer operating with the Cu Kα (λ = 154,056 Å) radiation. Nitrogen adsorption/desorption isotherms at 77 K were measured using Quadrasorb SI apparatus (Quantachrome Instruments, USA). Prior to the measurements samples were subjected to 24 h-long degassing carried out under high vacuum and at 290 °C. The specific surface area values were determined from multi-point adsorption branches of the attained isotherms, applying Brunauer-Emmet-Teller (BET) equation. The total surface area values (S total), external surface areas (S ext), and micropore areas (S micro), were calculated using αs method. The Dubinin–Radushkevich equation was used to calculate micropore volumes (VmicroDR) from adsorption branches of the isotherms. The total pore volumes (Vtot0.95) were determined from the amounts of nitrogen adsorbed at relative pressure p/po = 0.95. Finally, the volume of mesopores (Vmeso) could be calculated by subtracting the micropore volume from the total pore volume. In order to determine pore size distributions in mesopore range, the Barrett, Joyner, and Halenda (BJH) method was applied to the N2 adsorption data.
Temperature programmed desorption (TPD) was examined using thermal desorption spectrometer (TDS1200 apparatus, ESCO Ltd., Japan) equipped with a quadrupole mass spectrometer. Evolution of CO2 and CO was continuously monitored on the basis of m/z = 44 and m/z = 28 signals, respectively. The measurement was carried out under a high vacuum (pressure below 10−6 Pa) and sample was heated up to 1,000 °C at a heating rate of 60 °C/min. Prior to the TPD, sample was heated to 700 °C under argon gas flow.
Adsorption of phenol, Basic Red 18 dye (BR18), and Reactive Red 198 dye (RR198) onto obtained porous carbons was examined applying the batch method. For that purpose, flasks containing suitable amounts (0–50 mg) of an individual carbon material were filled with 100 cm3 of aqueous solution of either phenol (100 mg/dm3) or BR18 (30 mg/dm3), or RR198 (30 mg/dm3), and then were thermostated for 2 h at 30 °C, in a shaking water bath. Concentrations of each model contaminant were determined spectrophotometrically using Jasco V-530 UV–Vis spectrometer. In order to estimate concentration of phenol, BR18, and RR198, absorbances at λmax = 270 nm, λmax = 480 nm, and λmax = 516 nm were measured, respectively. The amount of an adsorbate adsorbed on activated carbons at equilibrium, q e , was calculated according to the following equation:
where V [dm3] is the volume of solution used in the adsorption experiment, C i and C e are the initial and equilibrium concentrations of the adsorbate (mg/dm3), respectively, and m denotes mass of the adsorbent (g) used.
3 Results and discussion
3.1 Pyrolysis of MgCO3/PET mixtures and pore creation mechanism
The XRD pattern presented in Fig. 1 has confirmed that heating of MgCO3/PET mixture to 850 °C resulted in obtaining carbonaceous material loaded with expected MgO. Besides the oxide, stoichiometric amounts of CO2 must be formed in situ from the carbonate. Taking into consideration results of our earlier works [18, 26], occurrence of analogous effects was suspected in the pyrolysed MgCO3/PET mixture. Hence, reaction between occluded CO2 and the char formed from PET contained in the pyrolysed mixture was considered as highly probable to proceed. In order to confirm these predictions, TPD from MgCO3/PET material preheated to 700 °C was measured. Obtained results are presented in Fig. 2.
The TPD spectra confirmed an intensive evolution of CO2 from ca. 60 °C to over 300 °C followed by a less intensive release of the gas at higher temperatures, to ca. 800 °C. In addition, intensive evolution of carbon monoxide (CO) could be confirmed at temperatures above 600 °C. The results presented in Fig. 2 are convergent with those obtained for both BMC/PET and Mg(OH)2/PET systems. For that reason, formation of CO at the high temperatures was assumed to be due to the reaction between the carbon material (char) and CO2 formed from MgCO3. In view of above discussion, MgCO3 was confirmed to be an another example of magnesium compound that is capable to create porosity in carbon materials according to the analogous mechanism as reported in details elsewhere [18].
3.2 Porosity and pore size distribution
Results of nitrogen adsorption and desorption for BMC/PET—and MgO/PET—based carbons were already presented in our earlier report [18]. Therefore, in this work we show (Fig. 3) only appropriate results acquired for carbon materials obtained from MgCO3/PET and Mg(OH)2/PET mixtures.
Nitrogen adsorption/desorption isotherms measured for porous carbons obtained from: a MgCO3/PET 70/30 mixture at different preparation temperatures, b MgCO3/PET mixtures with different weight ratios of the components, prepared at 850 °C, c Mg(OH)2/PET 70/30 mixture at different treatment temperatures, and d Mg(OH)2/PET mixtures with different weight ratios of the components, prepared at 850 °C
Shapes of all the measured isotherms are resultant from type I and IV profiles according to the IUPAC classification [27]. Hence, apart from well-developed microporosity, all the materials reveal significant adsorption in mesopores that is confirmed by distinct hysteresis loops at relative pressures above around 0.5. As a rule, adsorption of nitrogen at 77 K increases along with both temperature of the preparation process and loading of magnesium compounds in raw mixtures. This is particularly manifested for carbons obtained from MgCO3/PET mixtures. Nevertheless, the general trends are convergent with those observed for nanoporous carbons prepared from PET included in the mixture with BMC [18].
The textural parameters characterizing structure of the carbon materials, calculated from nitrogen adsorption/desorption isotherms, are listed in Table 1. Because this work summarizes adsorption behaviors of the materials prepared by our group from all the studied (Mg compound)/PET mixtures, pore parameters reported earlier for carbons obtained from MgO/PET and BMC/PET are also included.
Regardless of magnesium compounds and temperatures used for preparations, all the carbon materials reveal high specific surface area ranging from over 500 m2/g to nearly 2,000 m2/g, arising from both micropores and mesopores. Taking into account data listed in Tab. 1, several general observations could be drawn:
-
Pore parameters determined for carbons prepared at 550 °C are generally lower than those calculated for carbons prepared at higher temperatures,
-
The total areas revealed by nanoporous carbons prepared through pyrolysis of PET included in a mixture with decomposable magnesium is higher compared to values determined for carbons obtained from MgO/PET systems,
-
While carbons prepared from MgO/PET exhibit predominant microporous character, mesoporosity dominates in the carbon materials prepared using BMC, MgCO3, or Mg(OH)2,
-
Higher loadings of decomposable magnesium compounds in the raw mixtures are reflected by higher pore parameters of the resultant carbons,
-
Pore parameters determined for carbons prepared from MgO/PET mixtures are only a little influenced by MgO loadings in the starting mixtures and by preparation temperature.
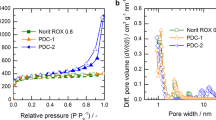
As illustrated in Fig. 4, pore size distributions in the mesopore region determined for porous carbons prepared from the MgCO3/PET mixtures are different compared to carbons prepared from PET loaded with Mg(OH)2. The mesopore volume calculated for MgCO3/PET-based carbons strongly depends on both MgCO3 content in the raw material and preparation temperature. This principally concerns mesopores ranging from 5 to ca. 25 nm in the width. In addition, the volume of mesopores within this width range depends noticeably on the preparation temperature. Hence, while the carbon prepared at 550 °C reveals low pore volume without a defined maxima, material obtained at 850 °C shows significant pore volume within the range, with two sharp maxima at pore widths ca. 8 and 20 nm.
On the other hand, the carbons prepared from PET mixed with Mg(OH)2 demonstrate predominant share of larger mesopores (15–40 nm in width) and lower contribution of the smaller ones, ranging from 5 to 15 nm in width. Although pore size distributions in the mesopore range vary with the composition of the raw mixture, no clear trend could be observed in this case. Nevertheless, similar to MgCO3/PET system, application of the higher temperature for preparations results in obtaining products demonstrating higher mesopore volumes. In view of corresponding results reported for carbon materials prepared from MgO/PET mixes [18], it may be stated that mesopore volumes revealed by both MgCO3/PET- and Mg(OH)2- series carbons are higher. This must be an outcome of the action of CO2 and H2O on the char during the pyrolysis process.
3.3 Adsorption of model contaminants
Because of considerable volumes of micropores and mesopores, the nanoporous carbons were tested as adsorbents for model water contaminants of a wide range of molecular weights. For that purpose aqueous solutions of phenol (94 g/mol), BR18 (390 g/mol), and RR198 (968 g/mol) were used as media conveying the adsorbates.
3.3.1 Removal of phenol from water
Adsorption of phenol on the obtained porous carbons is illustrated by Figs. 5 and 6.
All the examined adsorbents are to some extent capable to remove the contaminant from water. As it can be seen in Fig. 5a, b, regardless of MgCO3/PET and Mg(OH)2/PET ratios used for preparations, the resulting adsorbents reveal comparable adsorption abilities towards phenol, ranging from 81 to 106 mg/g. For comparison, adsorption of phenol on the carbons obtained from BMC/PET and MgO/PET mixtures is generally a little higher and ranges from nearly 70 to 114 mg/g. In addition, the removal efficiency tends to increase along with relative weight contents of magnesium compounds in the raw mixes, Fig. 5c, d. It is generally claimed [28] that adsorption of small molecules like phenol predominantly depends on the microporosity of sorbent materials. Hence, relatively low and comparable micropore areas and volumes (Table 1) determined for carbons prepared from MgCO3/PET and Mg(OH)2/PET mixes and generally higher parameters of two other series of carbons, clearly elucidate a cause of the observed trends.
As shown in Fig. 6, uptake of phenol by the porous carbons obtained from most of the mixtures increases along with the preparation temperature. Thus, while products produced at the lowest temperature, 550 °C, are of rather low adsorption capacity (40–60 mg/g), uptake of phenol by porous carbons prepared at 850 °C is considerably higher and ranges from 81 to 114 mg/g. In view of the data listed in Table 1, this tendency appears to be also due to the increase in micropore area and volume of the obtained adsorbents.
Adsorption capacity of various activated carbons towards phenol was reported as ranging from 35 to 150 mg/g [29–31]. Hence, performance of the carbon materials examined in this work can be considered as typical, however, strongly influenced by the preparation conditions.
3.3.2 Removal of Basic Red 18 from water
Performance of the obtained nanoporous carbons during adsorption of Basic Red 18 from water is illustrated by Figs. 7 and 8.
Taking into account the relatively poor effect (86–111 mg/g) revealed by the carbons obtained from MgO/PET mixtures and quite effective uptake (90–307 mg/g) of the dye by the other samples, some specific observations could be noticed. In general, uptake of the dye by carbons prepared through pyrolysis of (decomposable magnesium compound)/PET mixtures is superior compared to that determined for carbons obtained from MgO/PET mixes. Moreover, while impact of MgO/PET ratio on the BR18 removal is rather minor, performance of all the other materials substantially advances along with loadings of the inorganic components in the initial mixtures, Fig. 7. Above effect particularly concerns both MgCO3/PET and BMC/PET systems.
Taking into account textural parameters compiled in Table 1, distinct connection between the discussed trends and pore structure can be noticed. All the tested adsorbents demonstrate comparable micropore areas and volumes within a series. On the other hand, while the mesopore parameters determined for the MgO/PET—based carbons are comparable, mesopore areas and volumes revealed by the other carbons substantially increase along with (Mg compound)/PET ratio. The latter observation particularly concerns MgCO3/PET- and BMC/PET- series carbons and to a lower extent Mg(OH)2/PET—based ones. Hence, the mesoporosity is a key factor enhancing adsorption of the dye from aqueous solution.
Analogous trends could be found for results presented in Fig. 8. For instance, relatively poor performance of all the carbons prepared at 550 °C must be due to evidently low mesopore areas and volumes. On the other hand, similar effects revealed by BMC/PET or Mg(OH)2/PET—based samples prepared and 700 and 850 °C must be resulting from distinctly high and comparable mesopore parameters.
Compared to available literature data concerning adsorption of BR18 onto granular activated carbon [32], only adsorbent materials prepared from raw mixtures highly loaded with the decomposable magnesium compounds reveal comparable uptake of the dye. However, performance of the studied materials towards BR18 can be valued as superior if the effects exhibited by activated sludge (60 mg/g) [33] or by biosorbent prepared from tamarind hulls (75 mg/g) [34] are considered.
3.3.3 Removal of Reactive Red 198 from water
A series of measurements were carried out on the prepared carbons to examine adsorption of another dye, Reactive Red 198, from water. Collected results are illustrated by Figs. 9 and 10.
Despite divergent molecular weights of the dyes, the general trends observed during removal of the dye are in principle analogous to those observed for Basic Red 18. As illustrated on Fig. 9d, uptake of RR198 by MgO/PET—series nanocarbons is rather low, 10–34 mg/g, and quantitative composition of the raw MgO/PET mixtures used for preparations has a minor impact on the adsorption. On the other hand, effect of MgCO3, Mg(OH)2, and BMC shares in the raw mixtures on the adsorption of the dye is substantial. In each case, higher loadings of the Mg compounds in the raw mixtures are beneficial for obtaining porous carbons revealing higher uptake of the model contaminant, ranging from 24 to 115 mg/g. In view of data presented in Table 1 it may be stated that observed trends are concurrent with those already discussed for BR18. In this way, crucial role of mesoporosity in the RR198 removal from water is confirmed. Consequently, relatively low and comparable mesopore parameters determined for the adsorbents obtained from MgO/PET mixtures must be the reason of their poor performance, observed in spite of relatively high micropore parameters determined for these materials.
An influence of temperature used during preparations on RR198 adsorption is illustrated by Fig. 10. Regardless of combination of raw materials, strong influence of mesopore area and volume (see Table 1) on RR198 removal from water could be confirmed. Hence, particularly high uptake of the contaminant by porous carbons prepared from MgCO3/PET (70/30, 850 °C) and from Mg(OH)2/PET (70/30, 850 °C) mixtures, 116 mg/g and 108 mg/g, respectively, can be attributed to very high mesopore areas and volumes. Consequently, low effect (8–30 mg/g) exhibited by carbon materials prepared at the lowest temperature, 550 °C, must be due to the lower mesopore parameters.
Findings discussed above confirm suitability of the studied materials for the removal of RR198 from water. This particularly concerns adsorbents prepared from PET mixed with decomposable Mg compounds. In comparison with results reported by others [35], RR198 uptake by the studied adsorbents can be estimated as superior compared to that measured for the char produced from sewage sludge. Nevertheless, MgO nanoparticles were reported [36] to adsorb the dye quite efficiently, 76 mg/g. This value can be considered as comparable to the effect revealed carbon materials prepared from PET in mixtures with BMC, Mg(OH)2, and MgCO3.
4 Conclusions
The present paper is principally aimed to investigate the suitability of carbon materials obtained from poly(ethylene terephthalate) in mixtures with different magnesium compounds, for adsorption of water contaminants of different molecular weights. As the novelty, both Mg(OH)2 and MgCO3 were confirmed to be suitable for preparation of porous carbon materials according to the procedure developed already for the BMC/PET systems. Thus, the choice of magnesium compounds capable to convert chars into highly porous carbons could be extended. The results presented confirmed capability of the studied carbons to adsorb various adsorbates including phenol, Basic Red 18, and Reactive Red 198. The pore structure of the adsorbents can be influenced by altering quantitative and qualitative composition of a mixture of raw materials or by varying temperature of the preparation process. Considering results of numerous tests, some hints supportive for planning preparation procedure to produce an adsorbent revealing optimal parameters for a specific application can be deduced. We believe the content of this work is an important practical complement for the more extensive research on nanoporous carbons prepared from PET contained in mixtures with various inorganic magnesium compounds.
Abbreviations
- PET:
-
Poly(ethylene terephthalate)
- BMC:
-
Light basic magnesium carbonate
- Decomp.:
-
Decomposition
- RR198:
-
Reactive Red 198
- BR18:
-
Basic Red 18
- XRD:
-
X-ray diffraction
- TPD:
-
The temperature programmed desorption
References
B.H. Hameed, A.A. Rahman, J. Hazard. Mater. 160, 576 (2008)
F.C. Wu, P.H. Wu, R.L. Tseng, R.S. Juang, J. Environ. Manag. 92, 708 (2011)
M.A. Lillo-Ródenas, A.J. Fletcher, K.M. Thomas, D. Cazorla-Amorós, A. Liares-Solano, Carbon 44, 1455 (2006)
S. Sumathi, S. Bhatia, K.T. Lee, A.R. Mohamed, Chem. Eng. J. 162, 194 (2010)
Q. Wen, C. Li, Z. Cai, W. Zhang, H. Gao, L. Chen, G. Zeng, X. Shu, Y. Zhao, Bioresour. Technol. 102, 942 (2011)
A. Silvestre-Albero, J. Silvestre-Albero, A. Sepulveda-Escribano, F. Rodriguez-Reinoso, Microporous Mesoporous Mater. 120, 62 (2009)
T. Morishita, T. Tsumura, M. Toyoda, J. Przepiórski, A.W. Morawski, H. Konno, M. Inagaki, Carbon 48, 2690 (2010)
M. Benadjemia, L. Milliere, L. Reinert, N. Benderdouche, L. Duclaux, Fuel Process. Technol. 92, 1203 (2011)
J.M.V. Nabis, C. Laginhas, P.J.M. Carrott, R. Carrott, J. Anal. Appl. Pyrolysis 87, 8 (2010)
K. Yang, J. Peng, H. Xia, L. Zhang, C. Srinivasakannan, S. Guo, J. Taiwan Inst, Chem. Eng. 41, 367 (2010)
A. Arami-Niya, W.M.A. Wan Daud, F.S. Mijalli, Chem. Eng. Res. And Des. 89, 657 (2011)
S.Z. Mohammadi, M.A. Karimi, D. Afzali, F. Mansouri, Desalination 262, 86 (2010)
Y. Li, Q. Du, X. Wang, P. Zhang, D. Wang, Z. Wang, Y. Xia, J. Hazard. Mater. 183, 583 (2010)
E.N. El Qada, S.J. Allen, G.M. Walker, Chem. Eng. J. 142, 1 (2008)
A. Ould-Idriss, M. Stitou, E.M. Cuerda-Correa, C. Fernández-González, A. Macías-García, M.F. Alexandre-Franco, V. Gómez-Serrano, Fuel Process. Technol. 92, 261 (2011)
J.N. Sahu, J. Acharya, B.C. Meikap, Bioresour. Technol. 101, 1974 (2010)
M.C. Almazán-Almazán, M. Pérez-Mendoza, M. Domingo-García, I. Fernández-Morales, F.J. López, F.J. López-Garzón, Fuel Process. Technol. 91, 236 (2010)
J. Przepiórski, J. Karolczyk, K. Takeda, T. Tsumura, M. Toyoda, A.W. Morawski, Ind. Eng. Chem. Res. 48, 7110 (2009)
J.B. Parra, C.O. Ania, A. Arenillas, F. Rubiera, J.J. Pis, Appl. Surf. Sci. 238, 304 (2004)
M. Inagaki, H. Miura, H. Konno, J. Eur. Ceram. Soc. 18, 1011 (1998)
M. Inagaki, S. Kobayashi, F. Kojin, N. Tanaka, T. Morishita, B. Tryba, Carbon 42, 3153 (2004)
O. Terakado, M. Hirasawa, J. Anal. Appl. Pyrolysis 73, 248 (2005)
T. Morishita, Y. Soneda, T. Tsumura, M. Inagaki, Carbon 44, 2360 (2006)
L. Yan, J. Zhuang, X. Sun, Z. Deng, Y. Li, Mater. Chem. Phys. 76, 119 (2002)
G. Helou, S.A. Tariq, Thermochim. Acta 228, 123 (1993)
J. Przepiórski, J. Karolczyk, T. Tsumura, M. Toyoda, M. Inagaki, A.W. Morawski, J. Therm. Anal. Calorim. 107, 1147 (2012)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquérol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
S.I. Kim, T. Yamamoto, A. Endo, T. Ohmori, M. Nakaiwa, Microporous Mesoporous Mater. 96, 191 (2006)
B. Ozkaya, J. Hazard. Mater. B129, 158 (2006)
J. Przepiórski, J. Hazard. Mater. B135, 453 (2006)
P. Ariyadejwanich, W. Tanthapanichakoon, K. Nakagawa, S.R. Mukai, H. Tamon, Carbon 41, 157 (2003)
B. Noroozi, G.A. Sorial, H. Bahrami, M. Arami, Dye. Pigment. 76, 784 (2008)
O. Gulnaz, A. Kaya, F. Matyar, B. Arikan, J. Hazard. Mater. B108, 183 (2004)
S. Khorramfar, N.M. Mahmoodi, M. Arami, K. Gharanjig, Color. Technol. 126, 261 (2010)
C. Jindarom, V. Meeyoo, B. Kitiyanan, T. Rirksomboon, P. Rangsunvigit, Chem. Eng. J. 133, 239 (2007)
G. Moussavi, M. Mahmoudi, J. Hazard. Mater. 168, 806 (2009)
Acknowledgments
This study was supported by the Polish Ministry of Science and Higher Education, Grant No. N R050004 10. The authors wish to thank Boruta–Kolor for the dyes used in this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Karolczyk, J., Janus, M. & Przepiórski, J. Removal of model contaminants from water by porous carbons obtained through carbonization of poly(ethylene terephthalate) mixed with some magnesium compounds. J Porous Mater 20, 159–170 (2013). https://doi.org/10.1007/s10934-012-9585-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-012-9585-y