Abstract
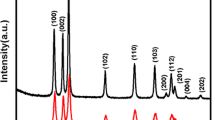
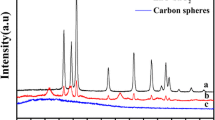
Porous zinc oxide (ZnO) hollow spheres have been fabricated by calcination of a precursor complex in a furnace. The precursor was precipitated in a chemical solution at 80 °C. The field-emission scanning electron microscope and transmission electron microscope reveal the porous and hollow structure of the samples. The spherical hollow precursor is self-assembled in the solution under the coordination effect of citrate ions and the Kirkendall effect working together. The precursors can be converted to pure ZnO crystals by heating in a furnace above 300 °C. The Brunauer–Emmett–Teller (BET) specific surface area of this sample is 95.4 m2/g. The photocatalytic degradation of methyl blue solution test shows the ZnO hollow spheres have superior photocatalytic activity.








Similar content being viewed by others
References
F. Caruso, R.A. Caruso, H. Mohwald, Science 282, 1111 (1998). doi:10.1126/science.282.5391.1111
Q. Peng, Y. Dong, Y. Li, Angew. Int. Ed. 42, 3027 (2003)
J. Bertling, J. Blomer, R. Kummel, Chem. Eng. Technol. 27, 8290 (2004). doi:10.1002/ceat.200406138
D. Zhang, L. Qi, J. Ma, H. Cheng, Adv. Mater. 14, 1499 (2002). doi:10.1002/1521-4095(20021016)14:20<1499::AID-ADMA1499>3.0.CO;2-5
D.Y. Wang, F. Caruso, Chem. Mater. 14, 1909 (2002). doi:10.1021/cm0211251
S.L. Xiong, J.M. Shen, Q. Xie, Q. Gao, Q. Tang, Y.T. Qian, Adv. Funct. Mater. 15, 1787 (2005). doi:10.1002/adfm.200500069
Y.Z. Li, T. Kunitake, S. Fujikawa, J. Phys. Chem. B 110, 13000 (2006). doi:10.1021/jp061979z
X. Xu, S.A. Asher, J. Am. Chem. Soc. 126, 7940 (2004). doi:10.1021/ja049453k
G. Zhu, S. Qiu, O. Terasaki, Y. Wei, J. Am. Chem. Soc. 123, 7723 (2001). doi:10.1021/ja0158758
C.E. Fowler, D.D. Khushalani, S. Mann, Chem. Commun. (Camb.) 19, 2028 (2001). doi:10.1039/b104879c
Z.X. Wang, M. Chen, L.M. Wu, Chem. Mater. 20, 3251 (2008). doi:10.1021/cm8001223
L. Li, Y. Chu, Y. Liu, L. Dong, J. Phys. Chem. C 111, 2123 (2007). doi:10.1021/jp066664y
Z.L. Wang, J. Phys. Condens. Matter. 16, 829 (2004). doi:10.1088/0953-8984/16/25/R01
Z.W. Pan, Z.R. Dai, Z.L. Wang, Science 291, 1947 (2001). doi:10.1126/science.1058120
Z.R. Tian, J.A. Voigt, J. Liu, B. Mckenzie, M.J. Mcdermott, J. Am. Chem. Soc. 124, 12954 (2002). doi:10.1021/ja0279545
F. Li, Y. Ding, P. Gao, X. Xin, Z.L. Wang, Angew. Chem. Int. Ed. 43, 5238 (2004). doi:10.1002/anie.200460783
M. Wang, C.H. Ye, Y. Zhang, G.M. Hua, H.X. Wang, M.G. Kong, L.D. Zhang, J. Cryst. Growth 291, 334 (2006). doi:10.1016/j.jcrysgro.2006.03.033
Z.Q. Li, Y. Xie, Y.J. Xiong, R. Zhang, New J. Chem. 27, 1518 (2003). doi:10.1039/b304787c
Q. Peng, S. Xu, Z.B. Zhuang, X. Wang, Y.D. Li, Small 1, 216 (2005). doi:10.1002/smll.200400043
J.X. Duan, X.T. Huang, E.K. Wang, H.H. Ai, Nanotechnology 17, 1786 (2006). doi:10.1088/0957-4484/17/6/040
Y.D. Yin, R.M. Rioux, C.K. Erdonmez, S. Hughes, G.A. Somorjai, A.P. Alivisatos, Science 304, 711 (2004). doi:10.1126/science.1096566
A.D. Smigelskas, E.O. Kirkendall, Trans. AIME 171, 130 (1947)
C.E. Birchenall, J. Electrochem. Soc. 103, 619 (1956). doi:10.1149/1.2430174
Q. Wu, X. Chen, P. Zhang, Y. Han, X. Chen, Y. Yan, S. Li, Cryst. Growth Des. 8, 3010 (2008). doi:10.1021/cg800126r
D. Mondelaers, G. Vanhoyland, H. Van den Rul, J. D’Haen, M.K. Van Bael, J. Mullens, L.C. Van Poucke, Mater. Res. Bull. 37, 901 (2002). doi:10.1016/S0025-5408(02)00727-4
Acknowledgment
This work was financial supported by the Education Department of Guangxi Province (200708MS130) and Guilin University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Cao, X., Wang, L. et al. Template-free fabrication of porous zinc oxide hollow spheres and their enhanced photocatalytic performance. J Porous Mater 17, 79–84 (2010). https://doi.org/10.1007/s10934-009-9266-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-009-9266-7