Abstract
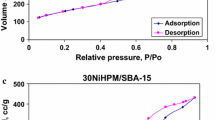
Two different mesoporous silicas (MesoPS) were synthesized by hydrothermal treatment in NaOH solution of two silica sources. One of these starting silicas was derived from selectively acid leached metakaolinite, and the other was from tetraethylorthosilicate (TEOS). The syntheses used a surfactant, cethyltrimethylammonium bromide (CTABr) and were carried out at different CTABr/Si, NaOH/Si and H2O/Si ratios. In the MesoPS from kaolinite, the specific surface areas (S BET) of the products were >1500 m2/g when prepared with 0.2 ≤ CTABr/Si ≤ 0.4, 0.3 ≤ NaOH/Si ≤ 0.6 and H2O/Si = 150. These S BET values are higher than those obtained from TEOS (ca. 1300 m2/g). The XRD patterns of these products contain a hexagonal (10-) peak with a lattice parameter a 0 = 4.2–5.2 nm in the MesoPS derived from kaolinite and a 0 = 4.0–4.6 nm in the products from TEOS. The regularity of the hexagonal structure is higher in the MesoPS derived from TEOS than from kaolinite. The pore size distributions of the products show a sharp peak at 2.8 nm in the MesoPS from kaolinite and at 2.4–2.5 nm in those from TEOS. The meso-structure is found to be formed during the stirring step of the synthesis and becomes more regular after hydrothermal treatment. The differences in the porous properties of the two MesoPSs from kaolinite and TEOS are attributed to differences in the dissolution rates and silica concentrations in the synthesis solutions. The 29Si MAS NMR spectra show a higher number of Q 3 (Si(OSi)3OH) units in the MesoPS from kaolinite and this is suggested to be related to the difference in their porous properties.








Similar content being viewed by others
References
T. Yanagisawa, T. Shimizu, K. Kuroda, C. Kato, Bull. Chem. Soc. Jpn. 63, 988 (1990). doi:10.1246/bcsj.63.988
C.T. Kresge, M.E. Loenowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 710 (1992). doi:10.1038/359710a0
A. Sayari, Stud. Surf. Sci. Catal. 102, 1 (1996). doi:10.1016/S0167-2991(06)81398-4
J.S. Beck, J.C. Vartuli, Curr. Opin. Solid State Mater. Sci. 1, 76 (1996). doi:10.1016/S1359-0286(96)80014-3
A.E.C. Palmqvist, Curr. Opin. Colloid Interface Sci. 8, 145 (2003). doi:10.1016/S1359-0294(03)00020-7
K. Okada, K.J.D. MacKenzie, in Nanomaterials from Research to Applications, ed. by H. Hosono, Y. Mishima, H.Takezoe, K.J.D. MacKenzie (Elsevier, Amsterdam, 2006), p. 349
K. Kosuge, K. Shimada, A. Tsunashima, Chem. Mater. 42, 717 (1995)
T. Shinoda, M. Onaka, Y. Izumi, Chem. Lett. 24, 495 (1995). doi:10.1246/cl.1995.495
K. Okada, H. Kawashima, Y. Saito, S. Hayashi, A. Yasumori, J. Mater. Chem. 5, 1241 (1995). doi:10.1039/jm9950501241
K. Okada, A. Shimai, T. Takei, S. Hayashi, A. Yasumori, K.J.D. MacKenzie, Microporous Mesoporous Mater. 21, 289 (1998). doi:10.1016/S1387-1811(98)00015-8
K. Okada, N. Nakazawa, Y. Kameshima, A. Yasumori, J. Temuujin, K.J.D. MacKenzie, M.E. Smith, Clays Clay Miner. 50, 623 (2002). doi:10.1346/000986002320679503
K. Okada, N. Yamamoto, Y. Kameshima, A. Yasumori, J. Colloid Interface Sci. 262, 179 (2003). doi:10.1016/S0021-9797(03)00107-3
J. Temuujin, K. Okada, K.J.D. MacKenzie, Appl. Clay Sci. 22, 187 (2003). doi:10.1016/S0169-1317(02)00158-8
K. Okada, N. Arimitsu, Y. Kameshima, A. Nakajima, K.J.D. MacKenzie, Appl. Clay Sci. 31, 185 (2006). doi:10.1016/j.clay.2005.10.014
K. Okada, K.J.D. MacKenzie, Curr. Topics Colloid Interface Sci. 7, 1 (2006)
C.D. Madhusoodana, Y. Kameshima, A. Yasumori, K. Okada, Clay Sci. 11, 369 (2001)
C.D. Madhusoodana, Y. Kameshima, A. Nakajima, K. Okada, T. Kogure, K.J.D. MacKenzie, J. Colloid Interface Sci. 297, 724 (2006). doi:10.1016/j.jcis.2005.10.051
S. Brunauer, P.H. Emmet, E. Teller, J. Am. Chem. Soc. 60, 309 (1938). doi:10.1021/ja01269a023
E.P. Barrett, L.G. Joyner, P.P. Halenda, J. Am. Chem. Soc. 73, 373 (1951). doi:10.1021/ja01145a126
Y. Cesteros, G.L. Haller, Microporous Mesoporous Mater. 43, 171 (2001). doi:10.1016/S1387-1811(00)00325-5
W. Stumm, J.J. Morgan, Aquatic chemistry (Wiley-Interscience, London, 1981)
K.J.D. MacKenzie, M.E. Smith, Multinuclear solid-state NMR of inorganic materials, vol. 6 (Pergamon Materials Series, Pergamon, Oxford, 2002)
Acknowledgment
We are grateful to Dr. C.D. Madhusoodana of Ceramic Technological Laboratory, BHEL, India, for fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, K., Yoshizaki, H., Kameshima, Y. et al. Porous properties of mesoporous silicas from two silica sources (acid-leached kaolinite and Si-alkoxide). J Porous Mater 17, 19–25 (2010). https://doi.org/10.1007/s10934-008-9260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-008-9260-5