Abstract
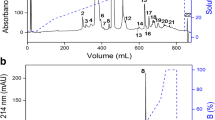
Disintegrins, a family of snake venom protein, which are capable of modulating the activity of integrins that play a fundamental role in the regulation of many physiological and pathological processes. The main purpose of this study is to obtain the recombinant disintegrin (r-DI) and evaluate its biological activity. In this study, we explored a high-level expression prokaryotic system and purification strategy for r-DI. Then, r-DI was treated to assay effects on cell growth, migration, and invasion. The affinity for the interactions of r-DI with integrin was determined using Surface plasmon resonance (SPR) analyses. The r-DI can be expressed in Escherichia coli and purified by one-step chromatography. The r-DI can inhibit B16F10 cells proliferation, migration, and invasion. Also, we found that r-DI could interact with the integrin αIIbβ3 (GPIIb/IIIa). The r-DI can be expressed, purified, characterized through functional assays, and can also maintain strong biological activities. Thus, this study showed potential therapeutic effects of r-DI for further functional and structural studies.









Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Hynes R, Ruoslahti E (2022) Reflections on Integrins-Past, Present, and future: the Albert Lasker Basic Medical Research Award [J]. JAMA 328(13):1291–1292
Luo B, Carman (2007) Structural basis of integrin regulation and signaling [J]. Annu Rev Immunol 25:619–647
Pandolfi F, Franza L (2017) Integrins: integrating the Biology and Therapy of Cell-cell interactions [J]. Clin Ther 39(12):2420–2436
Lo W, Lo S, Chen S S et al (2021) Molecular Imaging and Preclinical studies of Radiolabeled Long-Term RGD peptides in U-87 MG tumor-bearing mice [J]. Int J Mol Sci, 22(11)
Hu Q, Su Y (2024) MA S, et al. Integrin-targeted theranostic nanoparticles for clinical MRI-Traceable treatment of liver fibrosis [J], vol 16. ACS applied materials & interfaces, pp 2012–2026. 2
Men C, Zhang Y, Shi P et al (2023) ανβ3 integrin-targeted ICG-derived probes for imaging-guided surgery and photothermal therapy of oral cancer [J]. Analyst 148(24):6334–6340
Calvete J, Marcinkiewicz C, Monleón D et al (2005) Snake venom disintegrins: evolution of structure and function [J]. Toxicon: Official J Int Soc Toxinology 45(8):1063–1074
Tasoulis T, Isbister G (2022) A current perspective on snake venom composition and constituent protein families [J]. Archives of toxicology
Oliveira A, Viegas M, Da silva S et al (2022) The chemistry of snake venom and its medicinal potential [J]. Nat Reviews Chem 6(7):451–469
Angulo Y, Castro A, Lomonte B et al (2014) Isolation and characterization of four medium-size disintegrins from the venoms of Central American viperid snakes of the genera Atropoides, Bothrops, Cerrophidion and Crotalus [J]. Biochimie, : 376 – 84
Tonin G (2023) Klen J. Eptifibatide, an older therapeutic peptide with new indications: from clinical pharmacology to Everyday Clinical practice [J]. Int J Mol Sci, 24(6)
Arruda MacêdO J, Fox J, De souza castro M (2015) Disintegrins from snake venoms and their applications in cancer research and therapy [J]. Curr Protein Pept Sci 16(6):532–548
Vasconcelos A, Estrada J, Caruso I et al (2024) Toward the mechanism of jarastatin (rJast) inhibition of the integrin αVβ3 [J]. Int J Biol Macromol 255:128078
Zhou Q, Sherwin R, Parrish C et al (2000) Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits breast cancer progression [J]. Breast Cancer Res Treat 61(3):249–260
Kang I, Kim D, Jang Y et al (2000) Suppressive mechanism of salmosin, a novel disintegrin in B16 melanoma cell metastasis [J]. Biochem Biophys Res Commun 275(1):169–173
Yang R, Tang C, Chuang W et al (2005) Inhibition of tumor formation by snake venom disintegrin [J]. Toxicon: Official J Int Soc Toxinology 45(5):661–669
Chen J, Zhao J, Xie Z (2022) Integrin-mediated cancer progression as a specific target in clinical therapy [J], vol 155. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, p 113745
Fernandez J, Silva C, Assakura M et al (2005) Molecular cloning, functional expression, and molecular modeling of bothrostatin, a new highly active disintegrin from Bothrops jararaca venom [J]. Biochem Biophys Res Commun 329(2):457–464
Magalhães G, Novo J, Clissa P et al (2012) Engineered mammalian vector to express EGFP-tagged proteins as biomarkers [J]. Mol Biotechnol 51(2):119–127
Bose D, Roy L (2022) Peptide therapeutics in the management of metastatic cancers [J]. RSC Adv 12(33):21353–21373
Bialves T, Bastos junior C, Cordeiro M et al (2023) Snake venom, a potential treatment for melanoma. A systematic review [J]. Int J Biol Macromol 231:123367
Akhtar B, Muhammad F (2021) Mechanistic insights of snake venom disintegrins in cancer treatment [J]. Eur J Pharmacol 899:174022
Wermelinger L, Geraldo R, Frattani F et al (2009) Integrin inhibitors from snake venom: exploring the relationship between the structure and activity of RGD-peptides [J]. Arch Biochem Biophys 482:25–32
Bledzka K, Smyth S (2013) Integrin αIIbβ3: from discovery to efficacious therapeutic target [J]. Circul Res 112(8):1189–1200
Chew D, Bhatt D (2001) Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta-analysis of phase III multicenter randomized trials [J]. Circulation 103(2):201–206
Kuo Y, Chung C, Huang T. From Discovery of Snake Venom disintegrins to A Safer Therapeutic Antithrombotic Agent [J]. Toxins, 11(7)
Schönthal A, Swenson S, Chen T et al (2020) Preclinical studies of a novel snake venom-derived recombinant disintegrin with antitumor activity: a review [J]. Biochem Pharmacol 181:114149
Mcfadyen J, Schaff M (2018) Current and future antiplatelet therapies: emphasis on preserving haemostasis [J]. Nat Reviews Cardiol 15(3):181–191
Lazarovici P, Marcinkiewicz C (2019) Lelkes P. From Snake Venom’s Disintegrins and C-Type Lectins to Anti-Platelet Drugs [J]. Toxins, 11(5)
Doley R, Kini R (2009) Protein complexes in snake venom [J]. Cell Mol Life Sci 66(17):2851–2871
Singhamatr P (2007) Molecular cloning of albolatin, a novel snake venom metalloprotease from green pit viper (Trimeresurus albolabris), and expression of its disintegrin domain [J]. Toxicon: Official J Int Soc Toxinology 50(8):1192–1200
Sánchez E, Lucena S (2010) Cloning, expression, and hemostatic activities of a disintegrin, r-mojastin 1, from the mohave rattlesnake (Crotalus scutulatus scutulatus) [J]. Thromb Res 126(3):e211–e219
Zhang J, Zhang K, Ren Y et al (2021) The expression, purification, and functional evaluation of the novel tumor suppressor fusion protein IL-24-CN [J]. Appl Microbiol Biotechnol 105(20):7889–7898
Liu W, Zhang X, Song C et al (2014) Expression and characterization of a soluble VEGF receptor 2 protein [J]. Cell Bioscience 4(1):14
Studier F (2005) Protein production by auto-induction in high density shaking cultures [J]. Protein Exp Purif 41(1):207–234
Undefined U, Undefined U, Undefined U et al (2024) Immunotherapy in melanoma: can we predict response to treatment with circulating biomarkers? [J]. Pharmacol Ther, 256
Almeida G, De oliveira I, Arantese et al (2023) Snake venom disintegrins update: insights about new findings [J]. J Venom Anim Toxins Incl Trop Dis 29:e20230039
Offor B (2023) Piater L. Snake venom toxins: Potential anticancer therapeutics [J]. Journal of applied toxicology: JAT
Hailey S, Adams E, Penn R et al (2013) Effect of the disintegrin eristostatin on melanoma-natural killer cell interactions [J]. Toxicon: Official J Int Soc Toxinology 61:83–93
Undefined U, Undefined U, Undefined U et al (2012) Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) [J]. Toxicon, 60
Zou J, Swieringa F, De laat B et al (2022) Reversible platelet integrin αIIbβ3 activation and Thrombus instability [J]. Int J Mol Sci, 23(20)
Funding
This work was supported by Startup Fund for scientific research, Fujian Medical University(Grant number: 2022QH1216).
Author information
Authors and Affiliations
Contributions
Yinxiang Lan and Xiuliang Qiu wrote the main manuscript text, Yinxiang Lan and Xiuliang Qiu prepared figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Y., Qiu, X. & Xu, Y. Expression, Purification and Characterization of Recombinant Disintegrin from Gloydius Brevicaudus Venom in Escherichia Coli. Protein J (2024). https://doi.org/10.1007/s10930-024-10198-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s10930-024-10198-w