Abstract
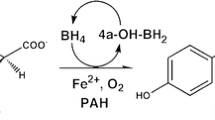
Phenylalanine ammonia lyase (PAL) catalyzes the deamination of phenylalanine to cinnamic acid and ammonia. It plays a crucial role in the formation of secondary metabolites through the phenylpropanoid pathway. Recently there has been growing interest in exploring the biochemical properties of PAL for its clinical and commercial applications. PAL as a key component has been used in metabolic engineering and synthetic biology. Due to its high substrate specificity and catalytic efficacy, PAL has opened a new area of interest in the biomedical field. PAL has been frequently used in the enzyme replacement therapy of phenylketonuria, cancer treatment and microbial production of l-phe the precursor of noncalorific sweetener aspartame (Methyl l-α-aspartyl-l-phenylalaninate), antimicrobial and health supplements. PAL occurs in few plants, fungi, bacteria, and cyanobacteria. The present investigation is a preliminary study in which an attempt has been made for the isolation, partial purification, and biochemical characterization of PAL (crude and partially purified) from Spirulina CPCC-695. Partially purified PAL exhibited higher enzymatic activity and protein content than the crude enzyme. Molecular weight of the crude and partially purified PAL was ~ 66 kDa. The optimum temperature and pH for PAL activity was observed as 30 ℃ and 8.0 respectively. l-Phe was the most preferred substrate (100 mM) whereas gallic acid showed maximum inhibition of PAL activity. Enzyme kinetics suggested good catalytic efficacy of the PAL enzyme and affinity towards substrate. Both the enzyme (crude and partially purified) showed less than 5% haemolysis suggesting the biocompatible nature of PAL.







Similar content being viewed by others
References
Xiang L, Moore BS (2005) Biochemical characterization of a prokaryotic phenylalanine ammonia lyase. J Bacteriol 187(12):4286–4289. https://doi.org/10.1128/jb.187.12.4286-4289.2005
Hyun MW, Yun YH, Kim JY, Kim SH (2011) Fungal and plant phenylalanine ammonia-lyase. Mycobiology 39(4):257–265. https://doi.org/10.5941/myco.2011.39.4.257
Fritz RR, Hodgins DS, Abell CW (1976) Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J Biol Chem 251(15):4646–4650. https://doi.org/10.1016/S0021-9258(17)33251-9
Bandoni RJ, Moore K, Rao PS, Towers GHN (1968) Phenylalanine and tyrosine ammonia-lyase activity in some Basidiomycetes. Phytochemistry 7(2):205–207. https://doi.org/10.1016/S0031-9422(00)86316-5
MacDonald MJ, D’Cunha GB (2007) A modern view of phenylalanine ammonia lyase. Biochem Cell Biol 85(3):273–282. https://doi.org/10.1139/o07-018
Evans CT, Conrad D, Hanna K, Peterson W, Choma C, Misawa M (1987) Novel stabilization of phenylalanine ammonia-lyase catalyst during bioconversion of trans-cinnamic acid to l-phenylalanine. Appl Microbiol Biotechnol 25(5):399–405. https://doi.org/10.1007/BF00253308
Hu GS, Jia JM, Hur YJ, Chung YS, Lee JH, Yun DJ, Kim DH (2011) Molecular characterization of phenylalanine ammonia lyase gene from Cistanche deserticola. Mol Biol Rep 38(6):3741–3750. https://doi.org/10.1007/s11033-010-0489-0
Ro DK, Douglas CJ (2004) Reconstitution of the entry point of plant phenylpropanoid metabolism in yeast (Saccharomyces cerevisiae): implications for control of metabolic flux into the phenylpropanoid pathway. J Biol Chem 279(4):2600–2607. https://doi.org/10.1074/jbc.m309951200
Cui JD, Qiu JQ, Fan XW, Jia SR, Tan ZL (2014) Biotechnological production and applications of microbial phenylalanine ammonia lyase: a recent review. Crit Rev Biotechnol 34(3):258–268. https://doi.org/10.3109/07388551.2013.791660
Hemmati S (2015) Phenylalanine ammonia-lyase through evolution: a bioinformatic approach. Trends Pharm Sci 1(1):10–14
Gilbert HJ, Jack GW (1981) The effect of proteinases on phenylalanine ammonia-lyase from the yeast Rhodotorula glutinis. Biochem J 199(3):715–723
Gilbert HJ, Clarke IN, Gibson RK, Stephenson JR, Tully M (1985) Molecular cloning of the phenylalanine ammonia lyase gene from Rhodosporidium toruloides in Escherichia coli K-12. J Bacteriol 161(1):314–320. https://doi.org/10.1128/jb.161.1.314-320.1985
Wang L, Gamez A, Archer H, Abola EE, Sarkissian CN et al (2008) Structural and biochemical characterization of the therapeutic Anabaena variabilis phenylalanine ammonia lyase. J Mol Biol 380(4):623–635. https://doi.org/10.1016/j.jmb.2008.05.025
Sarkissian CN, Gámez A (2005) Phenylalanine ammonia lyase, enzyme substitution therapy for phenylketonuria, where are we now? Mol Genet Metab 86:22–26
Moffitt MC, Louie GV, Bowman ME, Pence J, Noel JP, Moore BS (2007) Discovery of two cyanobacterial phenylalanine ammonia lyases: kinetic and structural characterization. Biochemistry 46(4):1004–1012. https://doi.org/10.1021/bi061774g
Löffelhardt W, Kindl H (1976) Formation of benzoic acid and p-hydroxybenzoic acid in the blue green alga Anacystis nidulans: a thylakoid-bound enzyme complex analogous to the chloroplast system. Zeitschrift für Naturforschung C 31(11–12):693–699. https://doi.org/10.1515/znc-1976-11-1212
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35(2):171–205. https://doi.org/10.1128/mmbr.35.2.171-205.1971
Chen K, Feng H, Zhang M, Wang X (2003) Nitric oxide alleviates oxidative damage in the green alga Chlorella pyrenoidosa caused by UV-B radiation. Folia Microbiol 48(3):389. https://doi.org/10.1007/bf02931372
Singh DP, Prabha R, Verma S, Meena KK, Yandigeri M (2017) Antioxidant properties and polyphenolic content in terrestrial cyanobacteria. 3 Biotech 7(2):1–14. https://doi.org/10.1007/s13205-017-0786-6
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Arafa A, Abdel-Ghani A, El-Dahmy S, Abdelaziz S, El-Ayouty Y, El-Sayed A (2020) Purification and characterization of Anabaena flos-aquae phenylalanine ammonia-lyase as a novel approach for myristicin biotransformation. J Microbiol Biotechnol. https://doi.org/10.4014/jmb.1908.08009
Lim HW, Park SS, Lim CJ (1997) Purification and properties of phenylalanine ammonia-lyase from leaf mustard. Mol Cells 7(6):410
Mongay CARLOS, Cerda V (1974) A Britton–Robinson buffer of known ionic strength. Ann Chim 64:409–412
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56(3):658–666
de Freitas Araújo MG, Hilário F, Vilegas W, Dos Santos LC, Brunetti IL, Sotomayor CE, Bauab TM (2012) Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int J Mol Sci 13(7):9260–9277. https://doi.org/10.3390/ijms13079260
Şirin S, Aydaş SB, Aslım B (2016) Biochemical evaluation of phenylalanine ammonia lyase from endemic plant Cyathobasis fruticulosa (Bunge) Aellen. For the dietary treatment of phenylketonuria. Food Technol Biotechnol 54(3):296–303
Jurado ECLV, Camacho F, Luzón G, Vicaria JM (2004) Kinetic models of activity for β-galactosidases: influence of pH, ionic concentration, and temperature. Enzyme Microb Technol 34(1):33–40. https://doi.org/10.1016/j.enzmictec.2003.07.004
Hou C, Yuan Q, Huo D, Zheng S, Zhan D (2008) Investigation on clotting and hemolysis characteristics of heparin-immobilized polyether sulfones biomembrane. J Biomed Mater Res Part A 85(3):847–852. https://doi.org/10.1002/jbm.a.31502
Koukol J, Conn EE (1961) The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J Biol Chem 236:2692–2698. https://doi.org/10.1016/S0021-9258(19)61721-7
Aydaş SB, Ozturk S, Aslım B (2013) Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem 136(1):164–169. https://doi.org/10.1016/j.foodchem.2012.07.119
Khan NU, Vaidyanathan CS (1986) A new simple spectrophotometric assay of phenylalanine ammonia-lyase. Curr Sci 184:391–393
Kovacs K, Banoczi G, Varga A, Szabo I, Holczinger A, Hornyanszky G, Poppe L (2014) Expression and properties of the highly alkalophilic phenylalanine ammonia-lyase of thermophilic Rubrobacter xylanophilus. PLoS ONE 9(1):e85943. https://doi.org/10.1371/journal.pone.0085943
Zhang F, Huang N, Zhou L, Cui W, Liu Z, Zhu L, Zhou Z (2017) Modulating the pH activity profiles of phenylalanine ammonia lyase from Anabaena variabilis by modification of center-near surface residues. Appl Biochem Biotechnol 183(3):699–711. https://doi.org/10.1007/s12010-017-2458-8
Kong JQ (2015) Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv 5(77):62587–62603
Ramsay RR, Tipton KF (2017) Assessment of enzyme inhibition: a review with examples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 22(7):1192
Alunni S, Cipiciani A, Fioroni G, Ottavi L (2003) Mechanisms of inhibition of phenylalanine ammonia-lyase by phenol inhibitors and phenol/glycine synergistic inhibitors. Arch Biochem Biophys 412(2):170–175. https://doi.org/10.1016/s0003-9861(03)00007-9
Gloge A, Langer B, Poppe L, Rétey J (1998) The behavior of substrate analogues and secondary deuterium isotope effects in the phenylalanine ammonia-lyase reaction. Arch Biochem Biophys 359(1):1–7. https://doi.org/10.1006/abbi.1998.0860
Sato T, Kiuchi F, Sankawa U (1982) Inhibition of phenylalanine ammonia-lyase by cinnamic acid derivatives and related compounds. Phytochemistry 21(4):845–850. https://doi.org/10.1016/0031-9422(82)80077-0
Lamb CJ, Rubery PH (1976) Phenylalanine ammonia-lyase and cinnamic acid 4-hydroxylase: product repression of the level of enzyme activity in potato tuber discs. Planta 130(3):283–290. https://doi.org/10.1007/BF00387834
Chen JY, He LH, Jiang YM, Wang Y, Joyce DC, Ji ZL, Lu WJ (2008) Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol Plant 32(3):318–328. https://doi.org/10.1111/j.1399-3054.2007.01013.x
Lam M, Scaman CH, Clemens S, Kermode A (2008) Retention of phenylalanine ammonia-lyase activity in wheat seedlings during storage and in vitro digestion. J Agric Food Chem 56(23):11407–11412. https://doi.org/10.1021/jf8021942
Okada T, Mikage M, Sekita S (2008) Molecular characterization of the phenylalanine ammonia-lyase from Ephedra sinica. Biol Pharm Bull 31(12):2194–2199. https://doi.org/10.1248/bpb.31.2194
Wen PF, Chen JY, Wan SB, Kong WF, Zhang P et al (2008) Salicylic acid activates phenylalanine ammonia-lyase in grape berry in response to high temperature stress. Plant Growth Regul 55(1):1–10. https://doi.org/10.3389/fpls.2015.00462
Louie GV, Bowman ME, Moffitt MC, Baiga TJ, Moore BS, Noel JP (2006) Structural determinants and modulation of substrate specificity in phenylalanine-tyrosine ammonia-lyases. Chem Biol 13(12):1327–1338. https://doi.org/10.1016/j.chembiol.2006.11.011
Hendrikse NM, Larsson AH, Gelius SS, Kuprin S, Nordling E, Syrén PO (2020) Exploring the therapeutic potential of modern and ancestral phenylalanine/tyrosine ammonia-lyases as supplementary treatment of hereditary tyrosinemia. Sci Rep 10(1):1–13. https://doi.org/10.1038/s41598-020-57913-y
Acknowledgements
Ahmad. R. sincerely thanks University Grants Commission (F.No. 61-1/2019 (SA-III)), India, for providing Maulana Azad National Fellowship (MANF-SRF). Authors are thankful to Culture Collection Centres of India, University of Madras, for providing the Spirulina CPCC-695 species.
Author information
Authors and Affiliations
Contributions
RA and TF designed the experiment, NS helped in the experiments and data curation. RA and GP carried out the lab work. RA wrote the initial manuscript that was corrected and finalised by TF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, R., Sami, N., Perveen, G. et al. Biochemical Characterization of Novel Phenylalanine Ammonia-Lyase from Spirulina CPCC-695. Protein J 41, 414–423 (2022). https://doi.org/10.1007/s10930-022-10063-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-022-10063-8