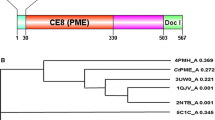
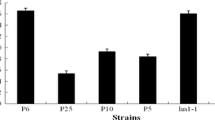
Abstract
Glucosinolates are plant natural products which on degradation by myrosinases give rise to the beneficial bioactive isothiocyanates. Recently, a myrosinase activity was detected in a Citrobacter strain isolated from soil. This enzyme was purified enabling its amino acid sequence and gene sequence (cmyr) to be determined. In order to study this myrosinase it was necessary to establish an expression system that would enable future work such as a structural determination of the protein to be carried out. The myrosinase gene was amplified, cloned and expressed in Escherichia coli with a 6XHis-tag. The heterologous expression of cmyr enabled relatively large amounts of myrosinase to be produced (3.4 mg cmyr/100 ml culture). Myrosinase activity was determined by mixing substrate and enzyme and determining glucose release. Optimum pH and temperature were determined to be pH 6.0 and 25 °C for the Ni-NTA purified protein. The kinetic parameters of the purified myrosinase were determined using sinigrin as a substrate. Km and Vmax were estimated as 0.18 mM and 0.033 mmol/min/mg respectively for sinigrin under optimum conditions and compared to other kinetic data for myrosinases. The substrate specificity of myrosinase was determined having the highest affinity for sinigrin followed by glucoiberin, progoitrin, glucoerucin, glucoraphanin and glucotropaeolin.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bones AM, Rossiter JT (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry. https://doi.org/10.1016/j.phytochem.2006.02.024
Kissen R, Rossiter JT, Bones AM (2009) The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev 8(1):69–86. https://doi.org/10.1007/s11101-008-9109-1
Mithen R, Ho E (2018) Isothiocyanates for human health. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201870079
Novío S, Cartea ME, Soengas P, Freire-Garabal M, Núñez-Iglesias MJ (2016) Effects of Brassicaceae isothiocyanates on prostate cancer. Molecules. https://doi.org/10.3390/molecules21050626
Chew FS (1988) Searching for defensive chemistry in the cruciferae, or, do glucosinolates always control ınteractions of cruciferae with their potential herbivores and symbionts? No! Chemical mediation of coevolution. Academic Press, pp 81–112. https://doi.org/10.1016/B978-0-12-656855-4.50008-5
Chew FS (1988) Biological effects of glucosinolates. Biologically active natural products. ACS Symposium Series, Vol. 380, pp 155–181. https://pubs.acs.org/doi/pdf/10.1021/bk-1988-0380.ch012
Rivera-Vega LJ, Krosse S, de Graaf RM, Garvi J, Garvi-Bode RD, van Dam NM (2015) Allelopathic effects of glucosinolate breakdown products in Hanza [Boscia senegalensis (Pers.) Lam.] processing waste water. Front Plant Sci 6:1–2. https://doi.org/10.3389/fpls.2015.00532
Neubauer C, Heitmann B, Müller C (2014) Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur J Plant Pathol 140(2):341–352. https://doi.org/10.1007/s10658-014-0467-9
Ngala BM, Haydock PP, Woods S, Back MA (2015) Biofumigation with Brassica juncea, Raphanus sativus and Eruca sativa for the management of field populations of the potato cyst nematode Globodera pallida. Pest Manag Sci 71(5):759–769. https://doi.org/10.1002/ps.3849
Sotelo T, Lema M, Soengas P, Cartea ME, Velasco P (2015) In vitro activity of glucosinolates and their degradation products against Brassica-pathogenic bacteria and fungi. Appl Environ Microbiol 81(1):432–440. https://doi.org/10.1128/AEM.03142-14
Wittstock U, Burow M (2007) Tipping the scales—specifier proteins in glucosinolate hydrolysis. IUBMB Life 59(12):744–751. https://doi.org/10.1080/15216540701736277
Geng F et al (2011) Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J Biol Chem 286(37):32259–32267. https://doi.org/10.1074/jbc.M111.278127
Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C (2005) Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer 117(3):356–362. https://doi.org/10.1002/ijc.21191
Burmeister WP, Cottaz S, Driguez H, Iori R, Palmieri S, Henrissat B (1997) The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase. Structure. https://doi.org/10.1016/s0969-2126(97)00221-9
Burmeister WP, Cottaz S, Rollin P, Vasella A, Henrissat B (2000) High resolution x-ray crystallography shows that ascorbate is a cofactor for myrosinase and substitutes for the function of the catalytic base. J Biol Chem 275(50):39385–39393. https://doi.org/10.1074/jbc.M006796200
Pontoppidan B, Ekbom B, Eriksson S, Meijer J (2001) Purification and characterization of myrosinase from the cabbage aphid (Brevicoryne brassicae), a brassica herbivore. Eur J Biochem 268(4):1041–1048. https://doi.org/10.1046/j.1432-1327.2001.01971.x
Jones AME, Bridges M, Bones AM, Cole R, Rossiter JT (2001) Purification and characterisation of a non-plant myrosinase from the cabbage aphid Brevicoryne brassicae (L.). Insect Biochem Mol Biol 31(1):1–5. https://doi.org/10.1016/S0965-1748(00)00157-0
Jones AME, Winge P, Bones AM, Cole R, Rossiter JT (2002) Characterization and evolution of a myrosinase from the cabbage aphid Brevicoryne brassicae. Insect Biochem Mol Biol 32(3):275–284. https://doi.org/10.1016/S0965-1748(01)00088-1
Francis F, Lognay G, Wathelet JP, Haubruge E (2002) Characterisation of aphid myrosinase and degradation studies of glucosinolates. Arch Insect Biochem Physiol 50(4):173–182. https://doi.org/10.1002/arch.10042
Husebye H et al (2005) Crystal structure at 1.1 Å resolution of an insect myrosinase from Brevicoryne brassicae shows its close relationship to β-glucosidases. Insect Biochem Mol Biol 35(12):1311–1320. https://doi.org/10.1016/j.ibmb.2005.07.004
Beran F et al (2014) Phyllotreta striolata flea beetles use host plant defense compounds to create their own glucosinolate-myrosinase system. Proc Natl Acad Sci USA 111(20):7349–7354. https://doi.org/10.1073/pnas.1321781111
Tani N, Ohtsuru M, Hata T (1974) Purification and general characteristics of bacterial myrosinase produced by Enterobacter cloacae. Agric Biol Chem 38(9):1623–1630. https://doi.org/10.1271/bbb1961.38.1623
Palop ML, Smiths JP, ten Brink B (1995) Degradation of sinigrin by Lactobacillus agilis strain R16. Int J Food Microbiol 26(2):219–229. https://doi.org/10.1016/0168-1605(95)00123-2
Meulenbeld GH, Hartmans S (2001) Thioglucosidase activity from Sphingo bacterium sp. strain OTG1. Appl Microbiol Biotechnol 56(5–6):700–706. https://doi.org/10.1007/s002530100726
Luang-In V, Narbad A, Nueno-Palop C, Mithen R, Bennett M, Rossiter JT (2014) The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol Nutr Food Res 58(4):875–883. https://doi.org/10.1002/mnfr.201300377
Ohtsuru M, Tsuruo I, Hata T (1969) Studies on fungous myrosinase. Agric Biol Chem 33(9):1309–1325. https://doi.org/10.1080/00021369.1969.10859462
Smits JP, Knol W, Bol J (1993) Glucosinolate degradation by Aspergillus clavatus and Fusarium oxysporum in liquid and solid-state fermentation. Appl Microbiol Biotechnol 38(5):696–701. https://doi.org/10.1007/BF00182812
Rakariyatham N, Butr-Indr B, Niamsup H, Shank L (2006) Improvement of myrosinase activity of Aspergillus sp. NR4617 by chemical mutagenesis. Electron J Biotechnol 9(4):341–347. https://doi.org/10.2225/vol9-issue4-fulltext-13
Narbad A, Rossiter JT (2018) Gut glucosinolate metabolism and ısothiocyanate production. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201700991
Mullaney JA, Ansell J, Kelly WJ, Heyes JA (2013) Biotransformation of glucosinolates from a bacterial perspective. CAB Rev: Perspect Agric Vet Sci Nutr Nat Resour. https://doi.org/10.1079/PAVSNNR20138034
Rouzaud G, Rabot S, Ratcliffe B, Duncan AJ (2003) Influence of plant and bacterial myrosinase activity on the metabolic fate of glucosinolates in gnotobiotic rats. Br J Nutr. https://doi.org/10.1079/bjn2003900
Elfoul L, Rabot S, Khelifa N, Quinsac A, Duguay A, Rimbault A (2001) Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol Lett 197(1):99–103. https://doi.org/10.1016/S0378-1097(01)00093-3
Albaser A et al (2016) Discovery of a bacterial glycoside hydrolase family 3 (GH3) β-glucosidase with myrosinase activity from a citrobacter strain isolated from soil. J Agric Food Chem 64(7):1520–1527. https://doi.org/10.1021/acs.jafc.5b05381
Liou CS et al (2020) A metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 180(4):717-728.e19. https://doi.org/10.1016/j.cell.2020.01.023
Bones AM, Rossiter JT (1996) The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol Plant 97(1):194–208. https://doi.org/10.1111/j.1399-3054.1996.tb00497.x
Bhat R, Vyas D (2019) Myrosinase: insights on structural, catalytic, regulatory, and environmental interactions. Crit Rev Biotechnol 39(4):508–523. https://doi.org/10.1080/07388551.2019.1576024
Sievers F et al (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol Syst Biol. https://doi.org/10.1038/msb.2011.75
Luang-In V (2013) Influence of human gut microbiota on the metabolic fate of glucosinolates. Imperial College London, UK
Bhat R, Kaur T, Khajuria M, Vyas R, Vyas D (2015) Purification and characterization of a novel redox-regulated isoform of myrosinase (β-Thioglucoside glucohydrolase) from Lepidium latifolium L. J Agric Food Chem 63(47):10218–10226. https://doi.org/10.1021/acs.jafc.5b04468
Chen S, Halkier BA (1999) Functional expression and characterization of the myrosinase MYR1 from Brassica napus in Saccharomyces cerevisiae. Protein Expr Purif 17(3):414–420. https://doi.org/10.1006/prep.1999.1158
Bridges M et al (2002) Spatial organization of the glucosinolate-myrosinase system in brassica specialist aphids is similar to that of the host plant. Proc R Soc B: Biol Sci. https://doi.org/10.1098/rspb.2001.1861
Kazana E et al (2007) The cabbage aphid: a walking mustard oil bomb. Proc R Soc B: Biol Sci. https://doi.org/10.1098/rspb.2007.0237
Li X, Kushad MM (2005) Purification and characterization of myrosinase from horseradish (Armoracia rusticana) roots. Plant Physiol Biochem 43(6):503–511. https://doi.org/10.1016/j.plaphy.2005.03.015
UK Soil Observatory. http://mapapps2.bgs.ac.uk/ukso/home.html. Accessed 15 Oct 2021
James DC, Rossiter JT (1991) Development and characteristics of myrosinase in Brassica napus during early seedling growth. Physiol Plant 82(2):163–170. https://doi.org/10.1111/j.1399-3054.1991.tb00076.x
Bernardi R, Finiguerra MG, Rossi AA, Palmieri S (2003) Isolation and biochemical characterization of a basic myrosinase from ripe Crambe abyssinica seeds, highly specific for epi-progoitrin. J Agric Food Chem 51(9):2737–2744. https://doi.org/10.1021/jf020796g
Sabaty M et al (2013) Detrimental effect of the 6 His C-terminal tag on YedY enzymatic activity and influence of the TAT signal sequence on YedY synthesis. BMC Biochem. https://doi.org/10.1186/1471-2091-14-28
Aslantas Y, Surmeli NB (2019) Effects of N-terminal and C-terminal polyhistidine tag on the stability and function of the thermophilic P450 CYP119. Bioinorg Chem Appl. https://doi.org/10.1155/2019/8080697
Lee YL, Su MS, Huang TH, Shaw JF (1999) C-terminal His-tagging results in substrate specificity changes of the thioesterase I from Escherichia coli. J Am Oil Chem Soc 76(10):1113–1118. https://doi.org/10.1007/s11746-999-0082-7
Cebeci F (2017) The metabolism of plant glucosinolates by gut bacteria. University of East Anglia, UK
Acknowledgements
We wish to thank to Dr. Gerhard Saalbach (John Innes Centre, Norwich) for his support to perform LCMS analysis.
Funding
Quadram Institute Bioscience is supported by the Biotechnological and Biological Sciences Research Council via Institute Strategic grants BB/J004529/1, BB/J004545/1, BB/R012490/1 and BB/R012512/1. Fatma Cebeci was supported by a scholarship funded by the Republic of Turkey Ministry of National Education.
Author information
Authors and Affiliations
Contributions
Conceptualization, JTR, RM and AN; methodology, FC and MM; validation, FC; formal analysis, FC; investigation, FC; resources, AN; writing—original draft preparation, FC; writing—review and editing, JTR, MM, RM and AN; visualization, FC and JTR; supervision, JTR, MM, RM and AN; project administration, AN; funding acquisition, AN. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cebeci, F., Mayer, M.J., Rossiter, J.T. et al. Molecular Cloning, Expression and Characterisation of a Bacterial Myrosinase from Citrobacter Wye1. Protein J 41, 131–140 (2022). https://doi.org/10.1007/s10930-021-10034-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-021-10034-5