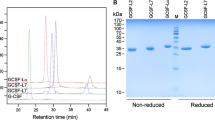
Abstract
Previously we reported that site-specific modification of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) A3C analog with polyethylene glycol (PEG) dramatically improved the pharmacokinetic properties of the protein in rats. However, we could not evaluate the hematological properties of the PEG-A3C protein in rats because human GM-CSF is inactive in rodents. To study the biological effects of PEGylated GM-CSF analogs in rodents we created a homologous site-specific PEGylated murine (mu) GM-CSF (T3C) protein. muGM-CSF and the T3C protein were expressed in Escherichia coli and purified by column chromatography. The purified T3C protein was covalently modified with a linear 20 kDa- or a branched 40 kDa-maleimide-PEG, and the monoPEGylated proteins purified by column chromatography. muGM-CSF, T3C and the two PEG-T3C proteins had comparable in vitro biological activities, as measured by stimulation of proliferation of the murine FDC-P1 cell line. The PEG-T3C proteins had 10- to 25-fold longer circulating half-lives than muGM-CSF and stimulated greater and longer lasting increases in neutrophils and white blood cells than muGM-CSF following a single intravenous or subcutaneous administration to rats. Treatment of rats made neutropenic with cyclophosphamide with the PEG-T3C proteins shortened the time for recovery of neutrophils to normal levels from 9 or 10 days to 5 or 6 days, whereas muGM-CSF showed no benefit versus vehicle solution. Acceleration of neutrophil recovery in cyclophosphamide-treated rats required a minimum of three PEG-T3C treatments over five days. The PEG-T3C proteins should prove useful for evaluating the potential therapeutic benefits of GM-CSF and long-acting GM-CSF proteins in rodent disease models.








Similar content being viewed by others
Abbreviations
- CBC:
-
Complete blood cell count
- CPA:
-
Cyclophosphamide
- EC50 :
-
Concentration causing half maximal response
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- Hu:
-
Human
- IV:
-
Intravenous
- Mu:
-
Murine
- PEG:
-
Polyethylene glycol
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SC:
-
Subcutaneous
- SD:
-
Standard deviation
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM:
-
Standard error of the mean
- TCEP:
-
Tris[2-carboxyethylphosphine]hydrochloride
- WBC:
-
White blood cell (total of lymphocytes, neutrophils, monocytes, eosinophils, and basophils)
References
Cebon JS, Lieschke GJ (1994) Granulocyte-macrophage colony-stimulating factor for cancer treatment. Oncology 51:177–188
Armitage JO (1998) Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491–4507
Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM (2014) Sargramostim plus ipilimumab vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312:1744–1753
Spitler LE, Weber RW, Allen RE, Meyer J, Cruickshank S, Garbe E, Lin H-Y, Soong S-J (2009) Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, Sargramostim) administered for 3 years as adjuvant therapy of stages II (T4), IIIand IV melanoma. J Immunother 32:632–637
Dranoff G (2002) GM-CSF-based cancer vaccines. Immunol Rev 188:147–154
Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S, Peoples GE (2012) Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 118:2594–2602
Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong T-H, Park H, Park HC, Park SR, Ha Y (2009) Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson’s disease model. Eur J Neurosci 29:891–900
Kong TH, Choi J-K, Park H, Choi BH, Snyder BJ, Bukjari S, Kim N-K, Huang X, Park SR, Park HC, Ha Y (2009) Reduction in programmed cell death and improvement in functional outcome of transient focal cerebral ischemia after administration of granulocyte-macrophage colony-stimulating factor in rats. J Neurosci 111:155–163
Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H (2010) GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimer’s Dis 21:507–518
Dieckgraefe BK, Korzenik JR (2002) Treatment of active Crohn's disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet 360:1478–1480
Gaudreau S, Guindi C, Ménard M, Besin G, Dupuis G, Amrani A (2007) Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 179:3638–3647
Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN (2011) GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol 240–241:65–73
Cheatem D, Ganesh BB, Gangia E, Vasu C, Prabhakar BS (2009) Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol 131:260–270
Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS (2009) GM-CSF-induced CD11c1CD8a—dendritic cells facilitate Foxp31 and IL-101 regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol 21:269–282
Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S (2009) Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol 162:478–486
Zhang L, Chen J, Han C (2009) A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns. Wound Repair Regen 17:685–689
Doherty DH, Rosendahl MS, Smith DJ, Hughes JM, Chlipala EA, Cox GN (2005) Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor. Bioconjug Chem 16:1291–1298
Gough NM, Gough J, Metcalf D, Kelso A, Grail D, Nicola NA, Burgess AW, Dunn AR (1984) Molecular cloning of a cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony-stimulating factor. Nature 309:763–767
Lee F, Yokota T, Otsuka T, Giemmell L, Larson N, Luh J, Arai K-I, Rennick D (1985) Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci USA 82:4360–4364
Kawasaki ES (1990) In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, San Diego
Picken RN, Mazaitis AJ, Maas WK, Rey M, Heyneker H (1983) Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect Immun 42:269–275
Rosendahl MS, Doherty DH, Smith DJ, Bendele AM, Cox GN (2005) Site-specific protein PEGylation: application to cysteine analogs of recombinant human granulocyte colony-stimulating factor. BioProcess Int 3:52–62
Smeaton TC (1984) Migration of polymorphonuclear neutrophils and macrophages from bone marrow to the peritoneal cavity after (3H)-thymidene labeling of rat tibial bone marrow in vivo. Aust J Exp Biol Med Sci 62:453–463
Lord BI, Molineux G, Pojda Z, Souza LM, Mermod J-J, Dexter TM (1991) Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte-colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood 77:2154–2159
Kuwabara T, Kato Y, Kobayashi S, Suzuki H, Sugiyama Y (1994) Nonlinear pharmacokinetics of a recombinant human granulocyte colony-stimulating factor derivative (nartograstim): species differences among rats, monkeys and humans. JPET 271:1535–1543
Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR (1999) Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA 96:1036–1041
Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, Maraskovsky E (2000) Polyethylene glycol-modified GM-CSF expands CD11bhigh CD11chigh but not CD11blow CD11chigh murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol 165:49–58
Sainathan KS, Tu L, Bishnupuri KS, Han M, Li A, Newberry RD, McDonald KG, Crimmins DL, Houchen C, Anant S, Dieckgraefe BK (2005) PEGylated murine granulocyte-macrophage colony-stimulating factor: production, purification and characterization. Protein Expr Purif 44:94–103
Cox GN, Chlipala EA, Smith DJ, Carlson SJ, Bell SJ, Doherty DH (2014) Hematopoietic properties of granulocyte colony-stimulating factor/immunoglobulin fusion proteins (G-CSF/IgG-Fc) in normal and neutropenic rodents. PLoS ONE 9(3):e91990. https://doi.org/10.1371/journal.pone.0091990
Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LA, Cox GN, Orschell CM (2014) PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys J 106:7–20
Acknowledgements
We thank Jennifer Hughes and Sharon Carlson for performing the ELISA and WinNonlin pharmacokinetic analyses.
Funding
This research was supported by Grant R44CA084850 from the National Cancer Institute to DHD. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or The National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GNC, DHD, MSR, and JIL are employees or former employees of Bolder BioTechnology. GNC and DHD have a financial interest in Bolder BioTechnology. GNC, DHD, and MSR are inventors on patents and patent applications owned by Bolder BioTechnology related to GM-CSF and other proteins and methods. EAC has a financial interest in Premier Laboratory, which was paid by Bolder BioTechnology to perform the animal studies described in the manuscript, as well as other animal studies.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Premier Laboratory and the University of Colorado, Boulder, where the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cox, G.N., Lee, J.I., Rosendahl, M.S. et al. Characterization of a Long-Acting Site-Specific PEGylated Murine GM-CSF Analog and Analysis of Its Hematopoietic Properties in Normal and Cyclophosphamide-Treated Neutropenic Rats. Protein J 39, 160–173 (2020). https://doi.org/10.1007/s10930-020-09894-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09894-0