Abstract
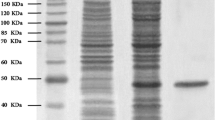
A 55 kDa cruciferin protein has been purified and characterized from seeds of Moringa oleifera plant. Protein blast of N-terminal amino-acid sequence showed 60 % sequence similarity with cruciferin from Brassica napus. The M. oleifera protein has been crystallized applying the sitting drop method using 5 % polyethylene glycol 8,000, 38.5 % 3-methyl-1,5-pentanediol and 0.1 M sodium cacodylate pH 6.5. The crystals belonged to the P6322 hexagonal space group with cell dimensions, a = b = 98.4, c = 274.3 Å. Initial diffraction data have been collected to a resolution of 6 Å.




Similar content being viewed by others
Abbreviations
- PEG:
-
Polyethylene glycol
- MPD:
-
3-Methyl-1,5-pentanediol
- MO:
-
Moringa oleifera
- DESY:
-
Deutsches Elektronen Synchrotron
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- DLS:
-
Dynamic light scattering
References
Ndabigengesere A, Narasiah KS, Talbot BG (1995) Active agents and mechanism of coagulation of turbid waters using Moringa oleifera. Water Res 29:703–710
Morton JF (1991) The horseradish tree, Moringa pterygosperma (Moringaceae). A boon to arid lands. Econ Bot 45:318–333
Grabow WOK, Slabert JL, Morgan WSG, Jahn SAA (1985) Toxicity and mutagenicity evaluation of water coagulated with Moringa oleifera seed preparations using fish, protozoan, bacterial, coliphage, enzyme, and Ames Salmonella assays. Water 11:9–14
D’souza J, Kulkarni AR (1993) Comparative studies on nutritive values of tender foliage of seedlings and mature plants of Moringa oleifera Lam. J Econ Taxon Bot 17:479–485
Anwar F, Ashraf M, Bhanger MI (2005) Interprovenance variation in the composition of Moringa oleifera oilseeds from Pakistan. J Am Oil Chem Soc 82:45–51
Anwar F, Bhanger MI (2003) Analytical characterization of Moringa oleifera seed oil grown in temperate regions of Pakistan. J Agric Food Chem 51:6558–6563
Wan L, Ross AR, Yang J, Hegedus DD, Kermode AR (2007) Phosphorylation of the 12 S globulin cruciferin in wild-type and abi1-1 mutant Arabidopsis thaliana (thale cress) seeds. Biochem J 404:247–256
Crouch ML, Sussex IM (1981) Development and storage-protein synthesis in Brassica napus L. embryos in vivo and in vitro. Planta 153:64–74
Hoeglund AS, Roedin J, Larsson E, Rask L (1992) Distribution of napin and cruciferin in developing rape seed embryos. Plant Physiol 98:509–515
Simon AE, Tenbarger KM, Scofield SR, Finkelstein RR, Crouch ML (1985) Nucleotide sequence of a cDNA clone of Brassica napus 12S storage protein shows homology with legumin from Pisum sativum. Plant Mol Biol 5:191–201
Roedin J, Rask L (1990) The relationship between mature chains and their precursors of cruciferin, the 12S storage protein of Brassica napus. Plant Sci 70:57–63
Gassenschmidt U, Jany KD, Tauscher B (1991) Chemical properties of flocculent—active proteins from Moringa oleifera lam. Biol Chem Hoppe-Seyler 372:659
Agrawal H, Shee C, Sharma AK (2007) Isolation of a 66 KDa protein with coagulation activity from seeds of Moringa oleifera. Global J Biotechnol Biochem 2:36–39
Santos AFS, Luz LA, Argolo ACC, Teixeira J, Paiva PMG, Coelho LCBB (2009) Isolation of a seed coagulant Moringa oleifera lectin. Process Biochem 44:504–508
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Roedin J, Sjoedahl S, Josefsson LG, Rask L (1992) Characterization of a Brassica napus gene encoding a cruciferin subunit: estimation of sizes of cruciferin gene families. Plant Mol Biol 20:559–563
Dalgalarrondo M, Robin JM, Azanza JL (1986) Subunit composition of the globulin fraction of rapeseed (Brassica napus L.). Plant Sci 43:115–124
Robin JM, Inquello V, Mimouni B, Azanza JL (1991) Relationship between immunological properties and structural model of 11 s rapeseed globulin. Phytochemistry 30:3511–3513
Bérot S, Compoint JP, Larré C, Malabat C, Guéguen J (2005) Large scale purification of rapeseed proteins (Brassica napus L.). J Chromatography B 818:35–42
Derbyshire E, Wright DJ, Boulter D (1976) Legumin and vicilin, storage proteins of legume seeds. Phytochemistry 15:3–24
Tandang MRG, Adachi M, Utsumi S (2004) Cloning and expression of rapeseed procruciferin in Escherichia coli and crystallization of the purified recombinant protein. Biotechnol Lett 26:385–391
Adachi M, Takenaka Y, Gidamis AB, Mikami B, Utsumi S (2001) Crystal structure of soybean proglycinin A1aB1b homotrimer. J Mol Biol 305:291–305
Matthews BW (1968) Solvent content of protein crystals. J Mol Biol 33:491–497
Acknowledgments
The work was supported by Higher Education Commission (HEC), Pakistan and Deutscher Akademischer Austausch Dienst (DAAD), Germany under the collaborative project “Ph.D scholarships for Engineering & Sciences in Germany’’ as a part of Human Resource Development (HRD) plan of Pakistan government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akrem, A., Yousef, N., Begum, A. et al. Preliminary Crystallographic Analysis of a Cruciferin Protein from Seeds of Moringa oleifera . Protein J 33, 253–257 (2014). https://doi.org/10.1007/s10930-014-9558-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-014-9558-x