Abstract
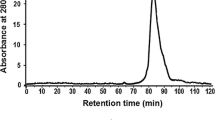
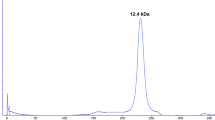
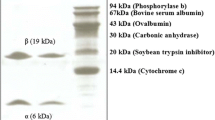
Lectins are proteins found in a wide range of organisms, with the ability to bind reversibly to specific carbohydrates. They can display important biological activities, such as the activation of the cell cycle in lymphocytes. Storage proteins with lectin activity have been reported in tuberous plant species, such as Colocasia esculenta, popularly known as taro. A simple strategy based on Cibacron Blue chromatography was used to purify a 12 kDa polypeptide 1.3-fold, with a recovery of 30 %. The purified protein was identified as tarin by mass spectrometry, which indicated that it was present in G1a/G1d isoforms. Tarin exhibited both agglutinating activity against hamster erythrocytes and mitogenic activity in vitro and in vivo toward mouse splenocytes. Optimum cellular proliferation in vitro was achieved by 625 ng of the crude extract or 500 ng of the purified tarin. Total mouse splenocyte proliferation measured after 5 days of intraperitoneal inoculation of purified tarin was increased 3.3-fold in comparison to the control group. Half of the proliferating cells were identified as B lymphocytes by flow cytometry. These results show that this is an efficient and simple strategy to purify tarin and aid in establishing this protein as a new therapeutic drug, able to promote cell proliferation in a murine model.




Similar content being viewed by others
Abbreviations
- [3H]-timidine:
-
(Tritiated)-thymidine
- Anti-IgM:
-
Anti-immunoglobulin M antibody
- BSA:
-
Bovine serum albumin
- Con A:
-
Concanavalin A
- FACS:
-
Fluorescence-activated cell sorter
- FCS:
-
Fetal calf serum
- FITC:
-
Fluorescein isothiocyanate
- GNA:
-
Galanthus nivalis agglutinin
- HIV:
-
Human immunodeficiency virus
- MALDI:
-
Matrix assisted laser desorption ionization
- PBS:
-
Phosphate buffered saline
- PE:
-
Phycoerythrin
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TOF:
-
Time of flight
References
Peumans WJ, Van Damme EJ (1995) Lectins as plant defense proteins. Plant Physiol 109(2):347–352
Carlini CR, Grossi-de-Sa MF (2002) Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 40(11):1515–1539
Vasconcelos IM, Oliveira JT (2004) Antinutritional properties of plant lectins. Toxicon 44(4):385–403
Rudiger H (1998) Plant lectins—more than just tools for glycoscientists: occurrence, structure, and possible functions of plant lectins. Acta Anat 161(1–4):130–152
Lis H, Sharon N (1998) Lectins: carbohydrate-specific proteins that mediate cellular recognition†. Chem Rev 98(2):637–674. doi:10.1021/cr940413g
Sharon N (2008) Lectins: past, present and future. Biochem Soc Trans 36(Pt 6):1457–1460
Michiels K, Van Damme EJ, Smagghe G (2010) Plant-insect interactions: what can we learn from plant lectins? Arch Insect Biochem Physiol 73(4):193–212
de Castro LA, Carneiro M, Neshich Dde C, de Paiva GR (1992) Spatial and temporal gene expression patterns occur during corm development. Plant Cell 4(12):1549–1559
Prajapati R, Kalariya M, Umbarkar R, Parmar S, Sheth N (2011) Colocasia esculenta: a potent indigenous plant. Int J Nutr Pharmacol Neurol Dis 1(2):90. doi:10.4103/2231-0738.84188
C-y Li, Meng L, Liu B, Bao J-k (2009) Galanthus nivalis agglutinin (GNA)-related lectins: traditional proteins, burgeoning drugs? Curr Chem Biol 3(3):323–333. doi:10.2174/187231309789054913
Bhat GG, Shetty KN, Nagre NN, Neekhra VV, Lingaraju S, Bhat RS, Inamdar SR, Suguna K, Swamy BM (2010) Purification, characterization and molecular cloning of a monocot mannose-binding lectin from Remusatia vivipara with nematicidal activity. Glycoconj J 27(3):309–320. doi:10.1007/s10719-010-9279-0
Shanmugham LN, Castellani ML, Salini V, Falasca K, Vecchiet J, Conti P, Petrarca C (2006) Relevance of plant lectins in human cell biology and immunology. Riv Biol 99(2):227–249
Krickeberg H, Mauff G, Mertens T, Plum G, Heitmann K (1990) Lymphocyte proliferation in AIDS-related complex/Walter-Reed 5 patients: response to herpes simplex virus and tuberculin antigen and mitogen during intravenous immunoglobulin treatment. The ARC-IVIG Study Group. Vox Sang 59(Suppl 1):38–43
Sharon N (2007) Lectins: carbohydrate-specific reagents and biological recognition molecules. J Biol Chem 282(5):2753–2764. doi:10.1074/jbc.X600004200
Zhang DL, Li LJ, Xia GT, He XY, Gao BX, Bai XH, Huang GS, Liu SG, Yan LF, Fang FD, Hu CL, Wang LJ, Jiang HH, Feng AM, Zhang GM, An SG, Ren YQ, Guo JM, Hu SX, Fan JX, Niu YL, Song ZJ, Li Y, Fan SJ (2001) Analyses of chromosomal karyotypes and cytogenetic variations of animal cell lines. Acta Genet Sinica 28(4):327–344
Yamamoto R, Azuma M, Kishida T, Yamada H, Satomura S, Fujimoto S (2001) Total alpha-fetoprotein and Lens culinaris agglutinin-reactive alpha-fetoprotein in fetal chromosomal abnormalities. Int J Obstet Gynaecol 108(11):1154–1158
Wimer BM (2003) Curative potential of foremost mitogen applications. Cancer Biother Radiopharm 18(6):903–916. doi:10.1089/108497803322702879
Roy A, Banerjee S, Majumder P, Das S (2002) Efficiency of mannose-binding plant lectins in controlling a homopteran insect, the red cotton bug. J Agric Food Chem 50(23):6775–6779
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice-Hall, Englewood Cliffs
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Schaffer AA, Aravind L, Madden TL, Shavirin S, Spouge JL, Wolf YI, Koonin EV, Altschul SF (2001) Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res 29(14):2994–3005
Van Damme EJ, Goossens K, Smeets K, Van Leuven F, Verhaert P, Peumans WJ (1995) The major tuber storage protein of araceae species is a lectin. Characterization and molecular cloning of the lectin from Arum maculatum L. Plant Physiol 107(4):1147–1158
Brown AC, Reitzenstein JE, Liu J, Jadus MR (2005) The anti-cancer effects of poi (Colocasia esculenta) on colonic adenocarcinoma cells in vitro. Phytother Res 19(9):767–771. doi:10.1002/ptr.1712
Xu HL, Li CY, He XM, Niu KQ, Peng H, Li WW, Zhou CC, Bao JK (2012) Molecular modeling, docking and dynamics simulations of GNA-related lectins for potential prevention of influenza virus (H1N1). J Mol Model 18(1):27–37
Bezerra IC, Castro LA, Neshich G, de Almeida ER, de Sa MF, Mello LV, Monte-Neshich DC (1995) A corm-specific gene encodes tarin, a major globulin of taro (Colocasia esculenta L. Schott). Plant Mol Biol 28(1):137–144
Carneiro M, Rodrigues CA, De Castro LAB, Da Silva MC, Coutinho MV (1990) Isolation characterization of the major albumin from Colocasia esculenta Corms. Plant Sci 67(1):39–46. doi:10.1016/0168-9452(90)90048-S
Monte-Neshich DC, Rocha TL, Guimarães RL, Santana EF, Loureiro ME, Valle M, Grossi de Sá MF (1995) Characterization and spatial localization of the major globulin families of taro (Colocasia esculenta L. Schott) tubers. Plant Sci 112(2):149–159. doi:10.1016/0168-9452(95)04257-1
Shewry PR (2003) Tuber storage proteins. Ann Bot 91(7):755–769. doi:10.1093/aob/mcg084
Hirai M, Nakamura K, Imai T, Sato T (1993) cDNAs encoding for storage proteins in the tubers of taro (Colocasia esculenta Schott). Idengaku zasshi 68(3):229–236
Einspahr H, Suguna K, Suddath FL, Ellis G, Helliwell JR, Papiz MZ (1985) The location of manganese and calcium ion cofactors in pea lectin crystals by use of anomalous dispersion and tuneable synchrotron X-radiation. Acta Crystallogr Sect B 41(5):336–341. doi:10.1107/S0108768185002233
Moreira Rde A, Ainouz IL, De Oliveira JT, Cavada BS (1991) Plant lectins, chemical and biological aspects. Mem Inst Oswaldo Cruz 86(Suppl 2):211–218
Sharon N (1993) Lectin-carbohydrate complexes of plants and animals: an atomic view. Trends Biochem Sci 18(6):221–226
Bryce RA, Hillier IH, Naismith JH (2001) Carbohydrate-protein recognition: molecular dynamics simulations and free energy analysis of oligosaccharide binding to concanavalin A. Biophys J 81(3):1373–1388. doi:10.1016/S0006-3495(01)75793-1
Tulin EE, Ecleo ZT (2007) Cytokine-mimetic properties of some Philippine food and medicinal plants. J Med Food 10(2):290–299. doi:10.1089/jmf.2006.067
Singh J, Kamboj SS (2004) A novel mitogenic and antiproliferative lectin from a wild cobra lily, Arisaema flavum. Biochem Biophys Res Commun 318(4):1057–1065. doi:10.1016/j.bbrc.2004.04.135
Kilpatrick DC (1999) Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol 11(1):55–65. doi:10.1007/BF02789176
Chen Y, Zhu B, Zhang L, Yan S, Li J (2009) Experimental study of the bone marrow protective effect of a traditional Chinese compound preparation. Phytother Res 23(6):823–826. doi:10.1002/ptr.2678
Takano F, Ohta Y, Tanaka T, Sasaki K, Kobayashi K, Takahashi T, Yahagi N, Yoshizaki F, Fushiya S, Ohta T (2009) Oral administration of Ren-Shen-Yang-Rong-Tang ‘Ninjin’yoeito’ protects against hematotoxicity and induces immature erythroid progenitor cells in 5-fluorouracil-induced anemia. Evidence-Based Complement Altern Med 6(2):247–256. doi:10.1093/ecam/nem080
Zhu XL, Zhu BD (2007) Mechanisms by which Astragalus membranaceus injection regulates hematopoiesis in myelosuppressed mice. Phytother Res 21(7):663–667
Acknowledgments
The present study was financially supported by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and by a Master’s degree scholarship provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We are very grateful for the collaboration with the Universidade Federal Fluminense, where all the biological experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, P.R., Del Aguila, E.M., Verícimo, M.A. et al. Purification and Characterization of the Lectin from Taro (Colocasia esculenta) and Its Effect on Mouse Splenocyte Proliferation In Vitro and In Vivo. Protein J 33, 92–99 (2014). https://doi.org/10.1007/s10930-013-9541-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9541-y