Abstract
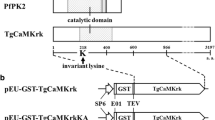
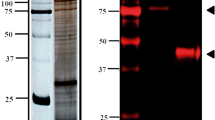
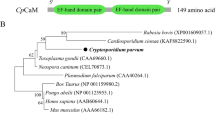
In trypanosomatids, Ca2+-binding proteins can affect parasite growth, differentiation and invasion. Due to their importance for parasite maintenance, they become an attractive target for drug discovery and design. Phytomonas serpens 15T is a non-human pathogenic trypanosomatid that expresses important protein homologs of human pathogenic trypanosomatids. In this study, the coding sequence of calmodulin, a Ca2+-binding protein, of P. serpens 15T was cloned and characterized. The encoded polypeptide (CaMP) displayed high amino acid identity to homolog protein of Trypanosoma cruzi and four helix-loop-helix motifs were found. CaMP sequence analysis showed 20 amino acid substitutions compared to its mammalian counterparts. This gene is located on a chromosomal band with estimated size of 1,300 kb and two transcripts were detected by Northern blot analysis. A polyclonal antiserum raised against the recombinant protein recognized a polypeptide with an estimated size of 17 kDa in log-phase promastigote extracts. The recombinant CaMP retains its Ca2+-binding capacity.




Similar content being viewed by others
Abbreviations
- CaMP:
-
Calmodulin from Phytomonas serpens 15T
- dNTP:
-
2′ Deoxynucleoside-5′ triphosphate
- EDTA:
-
Ethylene-diamine tetraacetic acid
- EF:
-
Hand helix-loop-helix structural domain
- EST:
-
Expressed sequence tag
- GYPMI:
-
Glucose, yeast extract, peptone, meat infusion culture medium
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
- SDS:
-
Sodium dodecyl sulphate
- TBE:
-
Tris-borate-EDTA buffer
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Nucleic Acids Res 25:3389–3402
Banerjee C, Sarkar D, Bhaduri A (1999) Parasitology 118:567–573
Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR (2004) Nucleic Acids Res 32:D138–D141
Batistoti M, Cavazzana MJr, Serrano MG, Ogatta SF, Baccan GC, Jankevicius JV, Teixeira MM, Jankevicius SI (2001) J. Parasitol 87:1335–1341
Benaim G, Lopez-Estraño C, Docampo R, Moreno SN (1993) Biochem J 296:759–763
Benaim G, Moreno SN, Hutchinson G, Cervino V, Hermoso T, Romero PJ, Ruiz F, de Souza W, Docampo R (1995) Biochem J 306:299–303
Benaim G, Cervino V, Villalobo A (1998) Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 120:57–65
Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araújo AP, Engman DM (2005) J Biol Chem 280:40104–40111
Camargo EP (1999) Adv Parasitol 42:29–112
Cheung WY (1980) Science 207:19–27
Chung SH, Swindle J (1990) Nucleic Acids Res 18:4561–4569
D’Angelo MA, Montagna AE, Sanguineti S, Torres HN, Flawiá MM (2002) J Biol Chem 277:35025–35034
Donovan C (1909) Lancet 177:1495–1496
Eid JE, Sollner-Webb B (1991) Genes Dev 5:2024–2032
Eisenberg D, Lüthy R, Bowie JU (1997) Methods Enzymol 277:396–404
Fragoso SP, Goldenberg S (1992) Mol Biochem Parasitol 55:127–134
Garcia-Marchan Y, Sojo F, Rodriguez E, Zerpa N, Malave C, Galindo-Castro I, Salerno M, Benaim G (2009) Exp Parasitol 123:326–333
Graça-de Souza VK, Monteiro-Góes V, Manque P, Souza TACB, Corrêa PRC, Buck GA, Ávila AR, Yamauchi LM, Pinge-Filho P, Goldenberg S, Krieger MA, Yamada-Ogatta SF (2010) Biol Res 43:233–241
Jankevicius JV, Itow-Jankevicius S, Campaner M, Conchon I, Maeda L, Teixeira MM, Freymuller E, Camargo EP (1989) J Eukaryot Microbiol 36:265–271
Laemmli UK (1970) Nature 227:680–685
Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL (1996) Exp Parasitol 83:240–249
Laskowski RA, Moss DS, Thornton JM (1993) J Mol Biol 231:1049–1067
Lu HG, Zhong L, Chang KP, Docampo R (1997) J Biol Chem 272:9464–9473
Maldonado RA, Linss J, Thomaz N, Olson CL, Engman DM, Goldenberg S (1997) Exp Parasitol 86:200–205
Moreno SN, Silva J, Vercesi AE, Docampo R (1994) J Exp Med 180:1535–1540
Moreno SN, Docampo R (2003) Curr Opin Microbiol 6:359–364
Nelson MR, Thulin E, Fagan PA, Forsén S, Chazin WJ (2002) Protein Sci 11:198–205
Ogueta SB, Solari A, Téllez-Iñón MT (1994) FEBS Lett 337:293–297
GJJr Pappas, Benabdellah K, Zingales B, González A (2005) Mol Biochem Parasitol 142:149–157
Porcel BM, Bontempi EJ, Henriksson J, Rydaker M, Aslund L, Segura EL, Pettersson U, Ruiz AM (1996) Exp Parasitol 84:387–399
Portman N, Lacomble S, Thomas B, McKean PG, Gull K (2009) J Biol Chem 284:5610–5619
Ridgley E, Webster P, Patton C, Ruben L (2000) Mol Biochem Parasitol 109:195–201
Rivarola HW, Bustamante JM, Lo Presti S, Fernández AR, Enders JE, Gea S, Fretes R, Paglini-Oliva P (2005) Exp Parasitol 111:80–86
Sambrook J, Russel DW (2001) Preparation and analysis of eukaryotic genomic DNA; Extraction, purification and analysis of mRNA from eukaryotic cells. In: Molecular cloning: a laboratory manual, 3rd ed, Cold Spring Harbor Laboratory, New York, USA
Santos AL, d’Ávila-Levy CM, Elias CG, Vermelho AB, Branquinha MH (2007) Microbes Infect 9:915–921
Serrano MG, Nunes LR, Campaner M, Buck GA, Camargo EP, Teixeira MM (1999) Exp Parasitol 91:268–279
Sivaprakasam P, Tosso PN, Doerksen RJ (2009) Structure-activity relationship and comparative docking studies or cycloguanil analogs as PfDHFR-TS inhibitors. J Chem Inf Model 49:1787–1796
Souza CF, Carneiro AB, Silveira AB, Laranja GA, Silva-Neto MA, Costa SC, Paes MC (2009) Biochem Biophys Res Commun 390:541–546
Téllez-Iñón MT, Ulloa RM, Torruella M, Torres HN (1985) Mol Biochem Parasitol 17:143–153
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49:3315–3321
Towbin H, Staehelin T, Gordon J (1979) Proc Natl Acad Sci USA 76:4350–4354
Trump BF, Berezesky IK (1995) FASEB J 9:219–228
Tschudi C, Ullu E (1988) EMBO J 7:455–463
Vickerman K (1994) Int J Parasitol 24:1317–1331
Wu Y, Deford J, Benjamin R, Lee MG, Ruben L (1994) Biochem J 304:833–841
Acknowledgments
We thank Paulo Lorca for help in camP nucleotide sequencing. We are also grateful to Dr. A. Leyva for reading this manuscript. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária and Pro-Reitoria de Pesquisa e Pós Graduação (PROPPG) of Universidade Estadual de Londrina (UEL). This study was part of the M.Sc. thesis work of T.A.C.B. Souza.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Arruda Campos Brasil de Souza, T., Graça-de Souza, V.K., Lancheros, C.A.C. et al. Identification, Molecular and Functional Characterization of Calmodulin Gene of Phytomonas serpens 15T that Shares High Similarity with its Pathogenic Counterparts Trypanosoma cruzi . Protein J 30, 212–219 (2011). https://doi.org/10.1007/s10930-011-9322-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9322-4