Abstract
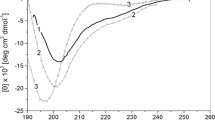
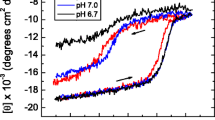
Recent advances in protein chemistry have led to progress in the understanding of protein folding and properties of possible intermediates during the folding of proteins. The molten globule (MG) state, a major intermediate of protein folding, has a denatured state with native-like secondary structure. In the present work, the acid-induced unfolding of wild type Escherichia coli 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS) and its three different variants (G96A, A183T and G96A/A183T) were studied by far- and near-UV circular dichroism (CD), intrinsic fluorescent emission spectroscopy and 1-anilino naphthalene-8-sulfonate (ANS) binding. At pH < 3.0, these EPSPS variants acquire partially folded state, which show the characteristics of the MG state, e.g., a drastic reduction of defined tertiary structure and almost no change in the secondary structure. ANS binding experiments show that hydrophobic surface of these variants is exposed to a greater extent in comparison to the native form, at acidic pH. Wild type, G96A, A183T and G96A/A183T acquire MG states at pH 2.0, 1.5, 3.0 and 3.0, respectively, which show that pH stability of MG state of G96A has increased in comparison to wild type; and pH stability of MG states of two other mutants is lower than that of the wild type. The results suggest that there is a direct relationship between stability of protein and pH stability of its folding intermediates.




Similar content being viewed by others
Abbreviations
- MG:
-
Molten globule
- EPSPS:
-
5-enolpyruvylshikimate 3-phosphate synthase
- CD:
-
Circular dichroism
- ANS:
-
1-anilino naphthalene-8-sulfonate
- DTT:
-
Dithiothreitol
- S3P:
-
Shikimate 3-phosphate
- MRW:
-
Mean amino acid residue weight
- Gdn-HCl:
-
Guanidine hydrochloride
References
Asghari SM, Khajeh K, Moradian F, Ranjbar B, Naderi-Manesh H (2004) Enzyme Microb Tech 57:35–51
Bentley R (1990) Crit Rev Biochem Mol Biol 25:307–383
Bradford MM (1976) Biochem 72:248–254
Chowdhury FA, Raleigh DP (2005) Protein Sci 14:89–96
D’Amico S, Marx JC, Gerday C, Feller G (2003) J Biol Chem 278:7891–7896
Eichholtz DA, Alan D, Gasser CS, Scott C, Kishore GM, Murthy G (2001) United States Patent No 6:114–225
Eschenburg S, Healy ML, Priestman MA, Lushington GH, Schönbrunn E (2002) Planta 216:129–135
Fitter J, Haber-Pohlmeier S (2004) Biochemistry 43:9589–9599
Goto Y, Takahashi N, Fink A (1990) Biochemistry 29:3480–3488
Goto Y, Fink AL (1989) Biochemistry 28:945–952
Haghani K, Salmanian AH, Ranjbar B, Zakikhan-Alang K, Khajeh K (2008) Biochim Biophys Acta 1784:1167–1175
Jagtap S, Rao M (2009) J Fluoresc 6:967–973
Kahrizi D, Salmanian AH, Afshari A, Moieni A, Mousavi A (2007) Plant Cell Rep 26:95–104
Kuwajima K (1989) Proteins 6:87–103
Lakowicz JR (1983) In principles of fluorescence spectreoscopy. Plenum, New York, pp 1991–2007
Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA (1979) Anal Biochem 100:95–97
Monsef Shokri M, Khajeh K, Alikhajeh J, Asoodeh A, Ranjbar B, Hosseinkhani S, Sadeghi M (2006) Biophys Chemistry 65:122–158
Nam GH, Choi KY (2002) Eur J Biochem 269:5280–5287
Protasevich I, Ranjbar B, Lobachov V, Markov A, Gilli D, Briand D, Lafitte J, Haiech R (1997) Biochemistry 36:2017–2024
Ptitsyn OB (1995) Adv Protein Chem 47:83–229
Schonbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN, Kabsch W (2001) Proc Natl Acad Sci USA 98:1376–1380
Steinrucken HC, Amrhein N (1980) Biochem Biophys Res Commun 94:1207–1212
Vieille C, Zeikus G (2001) Microbiol Mol Biol Rev 65:1–43
Wang HY, Li YF, Xie LX, Xu P (2003) J Plant Res 116:455–460
Weiss U, Mingioli ES (1956) J Biol Chem 78:2894–2898
Xie Q, Guo T, Lu J, Zhou HM (2004) Int J Biochem Cell Biol 36:296–306
Yang JT, Wu C, Martinez HM (1986) Methods Enzymol 130:208–278
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haghani, K., Khajeh, K., Salmanian, A.H. et al. Acid-Induced Formation of Molten Globule States in the Wild Type Escherichia coli 5-Enolpyruvylshikimate 3-Phosphate Synthase and its Three Mutated Forms: G96A, A183T and G96A/A183T. Protein J 30, 132–137 (2011). https://doi.org/10.1007/s10930-011-9308-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9308-2