Abstract
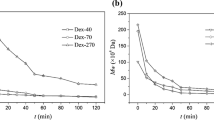
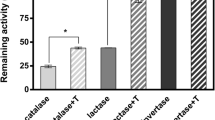
The influence of various concentration (10, 20, and 30% w/v) of different molar weighted dextrans as additives on the stability of HRP has been studied in aqueous medium. Native HRP preparations were formulated with different additives for storage stabilization and better performance at high temperature and pH. The results obtained show a stabilizing effect in the presence of an additive (75 kDa dextran). The enzyme with 75 kDa dextran (in concentration 10% w/v) showed the highest thermal resistance and the best performance for long-term storage at pH 5.0. In the presence of the 75 kDa dextran, the enzyme activity was increased threefold at 25 °C and lost only 15% activity in 2 h at 50 °C in comparison to the native enzyme which lost all its activity. In addition, dextran protected HRP against inactivation by air bubbles.








Similar content being viewed by others
Abbreviations
- HRP:
-
Horseradish peroxidase
- SPM:
-
Scanning probe microscope
- HPLC:
-
High performance liquid chromatography
References
Altikatoglu M, Arioz C, Basaran Y, Kuzu H (2009) Cent Euorpean J Chem 7:423–428
Altikatoglu M, Kuzu H (2010) Polish J Chem Technol 12:12–16
Arakawa T, Timasheff SN (1982) Biochemistry 21:6545–6552
Athes V, Combes D (1998) Enzyme Microb Technol 22:532–537
Back JB, Oakenfull D, Smith MB (1979) Biochemistry 18:5191–5196
Betancor L, López-Gallego F, Hidalgo A, Alonso-Morales N, Fuentes M, Fernández-Lafuente R, Guisán JM (2004) J Biotechnol 110:201–207
Costaa SA, Tzanova T, Carneiroa AF, Paarb A, Gubitzb GM, Cavaco-Paulo A (2002) Enzyme Microb Technol 30:387–391
Devi NA, Rao AGA (1998) J Agric Food Chem 46:3540–3545
Fagain O (1995) Biochem Biophys Acta 1252:1–14
Gekko K, Timasheff SN (1981) Biochemistry 20:4677–4686
Gibson TD, Higgins IJ, Woodward JR (1992) Analyst 117:1293–1297
Gouda MD, Singh SA, Appu Rao AG, Thakur MS, Karanth NG (2003) J Biol Chem 278:24324–24333
Graber M, Combes D (1989) Enzyme Microb Technol 11:673–677
Gulla KC, Gouda MD, Thakur MS, Karanth NG (2004) Biosens Bioelectron 19:621–625
Hassani L, Ranjbar B, Khajeh K, Naderi-Manesh H, Naderi-Manesh M, Sadeghi M (2006) Enzyme Microb Technol 38:118–125
Iyer PV, Ananthanarayan L (2008) Process Biochem 43:1019–1032
Ladero M, Santos A, Garcıa-Ochoa F (2006) Enzyme Microb Technol 38:1–9
Lenders JP, Crichton RR (1984) Biotechnol Bioeng 26:1343–1351
Longo MA, Combes D (1999) J Chem Technol Biotechnol 74:25–32
Michiaki M, Koji K, Kazuo K (1997) J Chem Technol Biotechnol 70:188–192
Mozhaev VV (1993) TIBTECH 11:88–95
Murakami Y, Hoshi R, Hirata A (2003) J Mol Catal B Enzym 22:79–88
Noel M, Combes D (2003) Enzyme Microb Technol 33:299–308
Renate UH, Ulrich AJM (1999) J Mol Catal B Enzym 7:125–131
Sadana A, Henley JP (1987) Biotechnol Bioeng 30:717–723
Santagapita PR, Brizuela LG, Mazzobre MF, Ramirez HL, Corti HR, Santana RV, Buera MP (2008) Biomacromolecules 9:741–747
Schmid RD (1979) Adv Biochem Eng 12:41–118
Swoboda BEP (1965) Biochim Biophys Acta 175:365–369
Acknowledgments
Authors wish to commemorate this study to beloved professor Huriye Kuzu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altikatoglu, M., Basaran, Y. Additive Effect of Dextrans on the Stability of Horseradish Peroxidase. Protein J 30, 84–90 (2011). https://doi.org/10.1007/s10930-011-9306-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-011-9306-4