Abstract
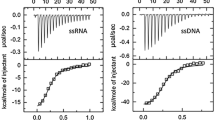
Single-stranded DNA-binding protein (SSB) plays an important role in DNA metabolism, such as in DNA replication, repair, and recombination, and is essential for cell survival. We characterized the single-stranded DNA (ssDNA)-binding properties of Pseudomonas aeruginosa PAO1 SSB (PaSSB) by using fluorescence quenching measurements and electrophoretic mobility shift analysis (EMSA). Analysis of purified PaSSB by gel filtration chromatography revealed a stable tetramer in solution. In fluorescence titrations, PaSSB bound 22–32 nucleotides (nt) per tetramer depending on salt concentration. Using EMSA, we characterized the stoichiometry of PaSSB complexed with a series of ssDNA homopolymers, and the size of the binding site was determined to be 29 ± 1 nt. Furthermore, EMSA results indicated that the dissociation constants of PaSSB for the first tetramer were less than those for the second tetramer. On the basis of these biophysical analyses, the ssDNA binding mode of PaSSB is expected to be noncooperative.







Similar content being viewed by others
Abbreviations
- Ec :
-
Escherichia coli
- Pa :
-
Pseudomonas aeruginosa PAO1
- Mt :
-
Mycobacterium tuberculosis
- Ms :
-
Mycobacterium smegmatis
- Hp :
-
Helicobacter pylori
- ssDNA:
-
Single-stranded DNA
- SSB:
-
Single-stranded DNA-binding protein
- SDS-PAGE:
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- EDTA:
-
Ethylenediamine tetraacetic acid
- EMSA:
-
Electrophoretic mobility shift analysis
- nt:
-
Nucleotides
- K d :
-
The apparent dissociation constant
References
Casas-Finet JR, Fischer KR, Karpel RL (1992) Proc Natl Acad Sci USA 89:1050–1054
Chan KW, Lee YJ, Wang CH, Huang H, Sun YJ (2009) J Mol Biol 388:508–519
Chase JW, Williams KR (1986) Annu Rev Biochem 55:103–136
Curth U, Genschel J, Urbanke C, Greipel J (1996) Nucleic Acids Res 24:2706–2711
Curth U, Greipel J, Urbanke C, Maass G (1993) Biochemistry 32:2585–2591
Dabrowski S, Olszewski M, Piatek R, Brillowska-Dabrowska A, Konopa G, Kur J (2002) Microbiology 148:3307–3315
Fairall L, Buttinelli M, Panetta G (2000) In: Travers A, Buckle M (eds) DNA-protein interactions: a practical approach. Oxford University Press, New York, pp 65–74
Fedorov R, Witte G, Urbanke C, Manstein DJ, Curth U (2006) Nucleic Acids Res 34:6708–6717
Genschel J, Litz L, Thole H, Roemling U, Urbanke C (1996) Gene 182:137–143
Haseltine CA, Kowalczykowski SC (2002) Mol Microbiol 43:1505–1515
Huang CY, Chang YW, Chen WT (2008) Biochem Biophys Res Commun 375:220–224
Huang CY, Hsu CH, Sun YJ, Wu HN, Hsiao CD (2006) Nucleic Acids Res 34:3878–3886
Jedrzejczak R, Dauter M, Dauter Z, Olszewski M, Dlugolecka A, Kur J (2006) Acta Crystallogr D Biol Crystallogr 62:1407–1412
Kelly TJ, Simancek P, Brush GS (1998) Proc Natl Acad Sci USA 95:14634–14639
Kerr ID, Wadsworth RI, Cubeddu L, Blankenfeldt W, Naismith JH, White MF (2003) EMBO J 22:2561–2570
Kim C, Snyder RO, Wold MS (1992) Mol Cell Biol 12:3050–3059
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Bioinformatics 23:2947–2948
Liu JH, Chang TW, Huang CY, Chen SU, Wu HN, Chang MC, Hsiao CD (2004) J Biol Chem 279:50465–50471
Lohman TM, Ferrari ME (1994) Annu Rev Biochem 63:527–570
Madden TL, Tatusov RL, Zhang J (1996) Methods Enzymol 266:131–141
Matos RG, Barbas A, Arraiano CM (2010) Protein J 29:394–397
Meyer RR, Laine PS (1990) Microbiol Rev 54:342–380
Murzin AG (1993) EMBO J 12:861–867
Oakley GG, Patrick SM (2010) Front Biosci 15:883–900
Olszewski M, Mickiewicz M, Kur J (2008) Arch Microbiol 190:79–87
Raghunathan S, Kozlov AG, Lohman TM, Waksman G (2000) Nat Struct Biol 7:648–652
Roy R, Kozlov AG, Lohman TM, Ha T (2007) J Mol Biol 369:1244–1257
Roy R, Kozlov AG, Lohman TM, Ha T (2009) Nature 461:1092–1097
Saikrishnan K, Jeyakanthan J, Venkatesh J, Acharya N, Sekar K, Varshney U, Vijayan M (2003) J Mol Biol 331:385–393
Saikrishnan K, Manjunath GP, Singh P, Jeyakanthan J, Dauter Z, Sekar K, Muniyappa K, Vijayan M (2005) Acta Crystallogr D Biol Crystallogr 61:1140–1148
Savvides SN, Raghunathan S, Futterer K, Kozlov AG, Lohman TM, Waksman G (2004) Protein Sci 13:1942–1947
Schwarz G, Watanabe F (1983) J Mol Biol 163:467–484
Shereda RD, Kozlov AG, Lohman TM, Cox MM, Keck JL (2008) Crit Rev Biochem Mol Biol 43:289–318
Tsai KL, Huang CY, Chang CH, Sun YJ, Chuang WJ, Hsiao CD (2006) J Biol Chem 281:17400–17409
Wadsworth RI, White MF (2001) Nucleic Acids Res 29:914–920
Wang CC, Tsau HW, Chen WT, Huang CY (2010) Protein J 29:445–452
Winsor GL, Lo R, Sui SJ, Ung KS, Huang S, Cheng D, Ching WK, Hancock RE, Brinkman FS (2005) Nucleic Acids Res 33:D338–D343
Witte G, Fedorov R, Curth U (2008) Biophys J 94:2269–2279
Witte G, Urbanke C, Curth U (2003) Nucleic Acids Res 31:4434–4440
Witte G, Urbanke C, Curth U (2005) Nucleic Acids Res 33:1662–1670
Wold MS (1997) Annu Rev Biochem 66:61–92
Yang C, Curth U, Urbanke C, Kang C (1997) Nat Struct Biol 4:153–157
Zhao WH, Hu ZQ (2010) Crit Rev Microbiol 36:245–258
Acknowledgments
We thank Ms. Hui-Chuan Hsieh for constructing the pET21b-PaSSB. This research was supported a grant from the National Research Program for Genome Medicine (NSC 99-3112-B-040-001 to C.Y. Huang).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jan, HC., Lee, YL. & Huang, CY. Characterization of a Single-Stranded DNA-Binding Protein from Pseudomonas aeruginosa PAO1. Protein J 30, 20–26 (2011). https://doi.org/10.1007/s10930-010-9297-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9297-6