Abstract
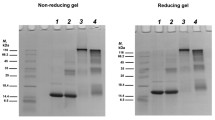
α-Crystallin functions as a molecular chaperone and maintains transparency of eye lens by protecting other lens-proteins. Non-enzymatic glycation of α-crystallin by methylglyoxal, plays a crucial role on its chaperone function and structural stability. Our studies showed that methylglyoxal modification even in lower concentration caused significant decrease in chaperone function of α-crystallin as reflected both in thermal aggregation assay and enzyme refolding assay. Thermal denaturation studies showed drastic reduction of denaturation temperature with increase in the degree of modification. Thermodynamic stability studies by urea denaturation assay reflected a decrease of transition midpoint. Quantitatively we found that ΔG° of native α-crystallin decreased from 21.6 kJ/mol to 10.4 kJ/mol due to 72 h modification by 10 mM methylglyoxal. The surface hydrophobicity of α-crystallin after MG modification, was found to be decreased. Circular dichroism spectroscopy revealed conversion of β-sheet structure to random coil structure. Significant cross-linking was also observed due to methylglyoxal modification of human α-crystallin.










Similar content being viewed by others
References
Ahmed N, Dobler D, Dean M, Thornalley PJ (2005) J Biol Chem 280:5724–5732
Ahmed N, Thornalley PJ, Dawczynski J (2003) Invest Opthalmol Vis Sci 44:5287–5292
Ahmed OK, Argirov HS, Minhas CA, Cordeiro A, Thornalley PJ (2002) Biochem J 364:1–14
Andley UP (2008) Int J Biochem Cell Biol 40(3):317–323
Babizhayev M, Deyev AI (1989) BBA 1004:124–133
Bhattacharyya J, Das KP (1998) Mol Biol Int 46:249–258
Biswas A, Das KP (2004) Protein Journal 23:529–538
Biswas A, Das KP (2004) J Biol Chem 279:42648–42657
Biswas A, Das KP (2008) Biochemistry 47:804–816
Biswas A, Lewis S, Wang B et al (2008) J Biochem 144(1):21–32
Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A (2004) Prog Biophys Mol Biol 86:407–485
Cardamone M, Puri BR (1992) Biochem J 282:589–593
Chaudhuri TK, Das KP, Sinha NK (1993) J Biochem 113(6):729–733
Cherian M, Abraham EC (1995) Biochem Biophys Res Comm 208:675–679
Das KP, Surewicz WK (1995) FEBS Lett 369:321–325
Derham BK, Harding JJ (2002) Biochem J 364:711–717
Eftink MR, Ghiron CA (1981) Ana Biochem 114:199
Farahbakhsh ZT, Huang QL, Ding LL, Altenbach C, Steinhoff HJ, Horwitz J, Hubbell WL (1995) Biochemistry 34:509–516
Gangadhariah MH, Wang B, Linetsky M, Henning C, Spanneberg R, Glomb MA, Nagaraj RH (2010) Biochim Biophys Acta 1802(4):432–441
Groenen PJTA, Merck KB, de Jong W, Bloemendal H (1994) Eur J Biochem 225:1–19
Haik GM, Lo TW, Thornalley PJ (1994) Exp Eye Res 59(4):497–500
Hanson SR, Hasan A, Smith DL, Smith JB (2000) Exp Eye Res 71:195–207
Horwitz J (1993) Invest Ophthalmol Vis Sci 34:10–21
Kantorow M, Piatigorsky J (1998) Int J Biol Macromol 22:307–314
Kumar MS, Mrudula T, Mitra N, Reddy GB (2004) Exp Eye Res 79:577–583
Kumar MS, Reddy PY, Kumar PA, Reddy GB (2004) Biochem J 379:273–282
Kundu M, Sen PC, Das KP (2007) Biopolymers 86(3):177–192
Lakowicz JR (1983) In: Principles of fluorescence spectroscopy. Plenum Press, New York
Lapolla A, Hamini R, Lupo A, Arico NC, Ruglu C, Reitano R, Tubaro K (2005) Ann N Y Acad Sci 1043:217–224
Lo TWC, Westwood ME, McLellan AC, Selwood T, Thornalley PJ (1994) J Biol Chem 269:32299–32305
Lowe J, McDermott H, Pike I, Spendlove I, Landon M, Mayer RJ (1992) J Pathol 166:61–68
Lyons TJ, Silvestri G, Dunn JA, Dyer DJ, Baynes JW (1991) Diabetes 40:1010–1015
McLellan AC, Thornalley PJ, Benn J, Sonksen PH (1994 Clin Sci (Lond) 87(1):21–29
Nagaraj RH, Oya-Ito T, Padayatti PS et al (2003) Biochemistry 42:10746–10755
Narberhaus F (2002) Microbiol Mol Biol Rev 66:64–93
Noritz J (1992) Proc Natl Acad Sci 89:10449–10453
Perry RE, Swamy MS, Abraham EC (1987) Exp Eye Res 44:269–282
Phillips SA, Mirrlees D, Thornalley PJ (1993) Biochem Pharmacol 46(5):805–811
Reddy GB, Das KP, Petrash JM, Surewicz WK (2000) J Biol Chem 275:4565–4570
Shamsi FA, Lin K, Sady C, Nagaraj RH (1998) Oopthalmol Vis Sci 39:2355–2364
Srinivasan AN, Nagineni CN, Bhat SP (1992) J Biol Chem 267:2333–2341
Stevens A, Augusteyn RC (1997) Eur J Biochem 243:792–797
Sun TX, Akhtar NJ, Liang JJN (1999) J Biol Chem 274:34067–34071
Surewicz WK, Olesen PR (1995) Biochemistry 34:9655–9660
Takemoto L, Sorensen CM (2008) Exp Eye Res 87:496–501
Thampi P, Hassan A, Smith JB, Abraham EC (2002) Invest Ophthalmol Vis Sci 43(10):3265–3272
Thampi P, Zarina S, Abraham EC (2002) Mol Cell Biochem 229(1–2):113–118
Thornalley PJ (1993) Mol Aspects Med 14:321–330
Thornalley PJ (2005) Ann N Y Acad Sci 1043:111–117
Ueda Y, Duncan MK, David LL (2002) Invest Ophthalmol Vis Sci 43:205–215
Verzijl N, DeGroot J, Ben ZC et al (2002) Arthritis Rheumatism 46(1):114–123
Ward RA, Mcleish KR (2004) Nephrol Dial Transplant 19(7):1702–1707
Zhang J, Yan H, Harding JJ, Liu ZX, Wang X, Ruan YS (2008) Curr Eye Res 33:963–976
Acknowledgments
The authors acknowledge with thanks the co-operation of Dr. Madhumita Das, Dept. of Opthalmology, NRS Medical College, Kolkata for supplying the human eye lenses. Continuous support of Dr. Madhuchanda Kundu and Sraboni Karmakar and the technical assistance of Dipak Konar are highly appreciated with thanks. The study is supported by the research grant of Department of Science & Technology, Government of India (Ref. No.: SR/WOS-A/LS- 414/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukhopadhyay, S., Kar, M. & Das, K.P. Effect of Methylglyoxal Modification of Human α-Crystallin on the Structure, Stability and Chaperone Function. Protein J 29, 551–566 (2010). https://doi.org/10.1007/s10930-010-9289-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9289-6