Abstract
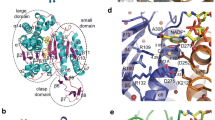
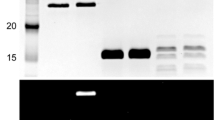
NADH oxidases (NOXs) are important enzymes in detoxifying oxidative stress and regenerating oxidized pyridine nucleotides. In the present study, a NOX from Thermococcus kodakarensis KOD1 (NOXtk) was recombinantly expressed in Escherichia coli and purified to homogeneity. NOXtk displayed NADH oxidase activity that was inhibited by oxidization. Under physiological conditions, unoxidized and oxidized NOXtk formed dimers and hexamers, respectively. Mutating the single cysteine residue Cys45 to alanine (NOXtkC45A) decreased NADH oxidase activity without affecting dimerization or hexamerization, suggesting that oligomerization does not occur through disulfide bond formation. Pull-down assay results indicated that an ATP/NAD kinase from T. kodakarensis KOD1 (ANKtk) binds to NOXtk. Use of several assays revealed that ANKtk can only bind to oxidized hexameric NOXtk, through which it inhibits ANKtk activity. Because ANKtk converts NADH to NADPH (an important factor in oxidative stress protection), a model based on in vitro result was proposed in which NOXtk hexamerization under oxic conditions inhibits both NOXtk and ANKtk activities, thereby sensitizing cells to oxidative stress-induced death.






Similar content being viewed by others
Abbreviations
- NOX:
-
NADH oxidase
- ANK:
-
ATP/NAD kinase
- native-PAGE:
-
Native polyacrylamide gel electrophoresis
- CBB:
-
Coomassie Brilliant Blue
- PVDF:
-
Poly(vinylidene difluoride)
- SDS–PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T (2004) Archaea 1:263–267
Banci L, Bertini I, Durazo A, Girotto S, Gralla EB, Martinelli M, Valentine JS, Vieru M, Whitelegge JP (2007) Proc Natl Acad Sci USA 104:11263–11267
Bieganowski P, Seidle HF, Wojcik M, Brenner C (2006) J Biol Chem 281:22439–22445
Caryn EO, Valeria CC (2008) EMBO J 22:2015–2024
Christopher MS, Dana HB, Eric JR (2008) Mol Microbiol 48:77–84
Dennis RH, Donald EW, Jeremy MF, Kyle ML, Ryan D, Murphy TCM, Edward JCI (2005) FEBS J 272:1189–1200
Donald EW, Christopher JD, Michael EM, John VDO, Willem MV, Edward JCI (2001) Eur J Biochem 268:5816–5823
Dym O, Eisenberg D (2001) Protein Sci 10:1712–1728
Giulio M, Giuseppe O, Nadia R (2006) Mini Rev Med Chem 6:739–746
Grose JH, Joss L, Velick SF, Roth JR (2006) Proc Natl Acad Sci USA 103:7601–7606
Jia B, Lee S, Pham BP, Cho Y, Yang J, Byeon H, Kim JC, Cheong G (2010) Mol Cells 29:1016–8478
Jia B, Park S, Lee S, Pham BP, Yu R, Le TL, Han S, Yang J, Choi M, Baumeister W, Cheong G (2008) FEBS J 275:5355–5366
Kawai S, Fukuda C, Mukai T, Murata K (2005) J Biol Chem 280:39200–39207
Kawai S, Mori S, Mukai T, Matsukawa H, Matuo Y, Murata K (2001) J Biosci Bioeng 92:447–452
Kawasaki S, Ishikura J, Chiba D, Nishino T, Niimura Y (2004) Arch Microbiol 181:324–330
Kengen SWM, van der Oost J, Vos WM (2003) Eur J Biochem 270:2885–2894
Labesse G, Douguet D, Assairi L, Gilles AM (2002) Trend Biochem Sci 27:273–275
Lerner F, Niere M, Ludwig A, Ziegler M (2001) Biochem Biophys Res Comm 288:69–74
Liu J, Lou Y, Yokota H, Adams PD, Kim R, Kim SH (2005) J Mol Biol 354:289–303
Modjtahedi N, Giordanetto F, Madeo F, Kroemer G (2006) Trend Cell Biol 16:264–272
Niimura Y, Nishiyam Y, Saito D, Tsuji H, Hidaka M, Miyaji T, Watanabe T, Massey V (2000) J Bacteriol 182:5046–5051
Pollak N, Niere M, Ziegler M (2007) J Biol Chem 282:33562–33571
Raffaelli N, Finaurini L, Mazzola F, Pucci L, Sorci L, Amici A, Magni G (2004) Biochemistry 43:7610–7617
Sakuraba H, Kawakami R, Ohshima T (2005) Appl Environ Microbiol 71:4352–4358
Shianna KV, Marchuk DA, Strand MK (2006) Mitochondrion 6:99–106
Shigeyuki K, Kousaku M (2008) Biosci Biotechnol Biochem 72:919–930
Shigeyuki K, Shigetarou M, Takako M, Wataru H, Kousaku M (2001) Eur J Biochem 268:4359–4365
Wu Y, Li Q, Chen XZ (2007) Nat Protocols 2:3278–3284
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Jia and S. Lee contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Jia, B., Lee, S., Pham, B.P. et al. Oxidized NADH Oxidase Inhibits Activity of an ATP/NAD Kinase from a Thermophilic Archaeon. Protein J 29, 609–616 (2010). https://doi.org/10.1007/s10930-010-9284-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-010-9284-y