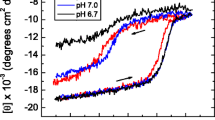
Abstract
Enolase is a multifunctional protein that participates in glycolysis and gluconeogenesis and can act as a plasminogen receptor on the cell surface of several organisms, among other functions. Despite its participation in a variety of biological and pathophysiological processes, its stability and folding/unfolding reaction have not been fully explored. In this paper we present, the urea and GdnHCl-induced denaturation of enolase studied by means of fluorescence and circular dichroism spectroscopies. We found that enolase unfolds through a highly reversible pathway, populating a stable intermediate species in a range of experimental conditions. The refolding reaction also exhibits an intermediate state that might have a slightly more compact conformation compared to the unfolding intermediate. The thermodynamic parameters associated with the unfolding reaction are presented and discussed.







Similar content being viewed by others
Abbreviations
- TRIS:
-
TRIS(hydroxy-methyl) aminomethane
- GdnHCl:
-
Guanidinium hydrochloride
- SCM:
-
Spectral center of mass
- CD:
-
Circular dichroism
- SASA:
-
Solvent-accessible surface area
References
Adamus G, Amundson D, Seigel GM, Machnicki M (1998) J Autoimmunol 11:671–677
Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA (1996) Clin Immunol Immunopathol 78:120–129
Akisawa N, Maeda T, Iwasaki S, Onishi S (1997) J Hepatol 26:845–851
Brewer JM (1969) Arch Biochem Biophys 134:59–66
Brewer JM, Faini GJ, Wu CA, Goss LP, Carreira LA, Wojcik R (1978) In: Catsim poolas N (ed) Physical aspects of protein interactions. Elsevier, North-Holland, p 57278
Brewer JM, Glover CVC, Holland MJ, Lebioda L (1997) Biochim Biophys Acta 1340:88–96
Brewer JM, Wampler JE (2001) Inter J Biol Macromol 28:213–218
Brown CK, Kuhlman PL, Mattingly S, Slates K, Calie PJ, Farrar WW (1998) J Protein Chem 17:855–866
Doyle SM, Braswell EH, Teschke CM (2000) Biochem 39:11667–11676
Fraczkiewicz R, Braun W (1998) J Comp Chem 19:319–333
Gawronski TH, Westhead EW (1969) Biochem 8:4261–4270
Gitlits VM, Sentry JW, Matthew MLSM, Smith AI, Toh BH (1997) Immunol 92:362–368
He P, Naka T, Serada S, Fujimoto M, Tanaka T, Hashimoto S, Shima Y, Yamadori T, Suzuki H, Hirashima T, Matsui K, Shiono H, Okumura M, Nishida T, Tachibana I, Norioka N, Norioka S, Kawase I (2007) Cancer Sci 98:1234–1240
Huang P, Dong A (2003) Spectroscopy 17:453–467
Kolberg J, Aase A, Bergmann S, Herstad TK, Rødal G, Frank R, Rohde M, Hammerschmidt S (2006) Microbiol 152:1307–1317
Kornblatt MJ, Al-Ghanim A, Kornblatt JA (1996) Eur J Biochem 236:78–84
Kornblatt MJ, Lange R, Balny C (2004) Eur J Biochem 271:3897–3904
Larsen TM, Wedekind JE, Rayment I, Reed GH (1996) Biochem 35:4349–4356
Lindquist S, Craig EA (1998) Annu Rev Genet 22:631–677
Mallam AL, Jackson SE (2005) J Mol Biol 346:1409–1421
Miller S, Janin J, Lesk AM, Chothia C (1987) J Mol Biol 196:641–656
Mixcoha-Hernández E, Moreno-Vargas LM, Rojo-Domínguez A, Benítez-Cardoza CG (2007) Protein J 26:491–498
Moodie FDL, Leaker B, Cambridge G, Totty NF, Segal AW (1993) Kidney Int 43:675–681
Myers JK, Pace CN, Scholtz JM (1995) Protein Sci 4:2138–2148
Najera H, Costas M, Fernandez-Velasco DA (2003) Biochem J 370:785–792
Orth T, Kellner R, Kiekmann O, Faust J, Meyer Zum Buschenfelde K-H, Mayet W-J (1998) Clin Exp Immunol 112:507–515
Nozaki Y (1972) Methods Enzymol 26:43–50
Pace CN (1986) Methods Enzymol 131:266–280
Pancholi V (2001) Cell Mol Life Sci 58:902–920
Peterson P, Perheentupa J, Krohn KJE (1996) Clin Diagn Lab Immunol 3:290–294
Petrak J, Ivanek R, Toman O, Cmejla R, Cmejlova R, Vyoral D, Zivny R, Vulpe CD (2008) Proteom 8:1744–1749
Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P (2000) J Rheumatol 27:109–115
Rattner JB, Martin L, Waisman DM, Johnstone SA, Fritzler MJ (1991) J Immunol 146:2341–2344
Roozendaal C, Zhao MH, Horst G, Lockwoods CM, Kleibeuker JH, Limburg PC (1998) Clin Exp Immunol 112:10–16
Rosenberg A, Lumry R (1964) Biochem 3:1055–1061
Rumfeldt JAO, Galvagnion C, Vassall KA, Meiering EM (2008) Progress Biophys Mol Bio 98:61–84
Schurig H, Rutkat K, Rachel R, Jaenicke R (1995) Protein Sci 4:228–236
Sedoris KC, Thomas SD, Miller DM (2007) Biochem 46:8659–8668
Tanford C (1970) Advan Protein Chem 24:1–95
Veronese FM, Schiavon O, Boccù E, Benassi CA, Fontana A (1984) Int J Pept Protein Res 24:557–562
Vick JE, Gerlt JA (2007) Biochem 18:14589–14597
Vora HK, Shaik FR, Pal-Bhowmick I, Mout R, Jarori GK (2009) Arch Biochem Biophys 485:128–138
Wang T, Himoe A (1974) J Biol Chem 249:3895–3902
Warren JR, Gordon JA (1966) J Phys Chem 70:297–300
Westhead EW (1964) Biochem 3:1062–1068
Zhao S, Choy BS, Kornblatt MJ (2008) FEBS J 275:97–106
Acknowledgments
We are very thankful with Dr. Andrés Hernández Arana for the use of the circular dichroism spectrometer. NCI was supported by a fellowship from CONACyT (204849) and from the Instituto Politécnico Nacional with a PIFI-IPN student grant. This work was supported by grants from TWAS (04-352 RG/BIO/LA), CONACyT (45990), and SIP-IPN (20080356, 20091074).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Miguel, D.S., Romero-Jiménez, J., Reyes-López, C.A. et al. Chemical Unfolding of Enolase from Saccharomyces cerevisiae Exhibits a Three-State Model. Protein J 29, 1–10 (2010). https://doi.org/10.1007/s10930-009-9215-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-009-9215-y