Abstract
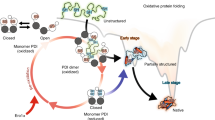
The isomerase efficacy of the oxidoreductase, protein disulfide isomerase (PDI), has been examined by a simple method. Using this technique, the pH-dependence of relative efficiency of isomerization reactions by PDI has been evaluated and its impact on a key structure-forming step in the oxidative folding pathway of a model protein determined. Results reveal that PDI has a greater relative impact on thiol-disulfide reshuffling (isomerization) reactions and consequently the structure-forming step in oxidative folding at pH 7, as opposed to pH’s 8 and 9. These results suggest that PDI, which possesses an anomalously low thiol pKa, is fine-tuned to catalyze oxidative folding in the lumen of the endoplasmic reticulum where the ambient pH of ∼7 would otherwise retard thiol-disulfide exchange reactions and hinder acquisition of the native fold. The pH-dependent impact on isomerization catalysis has important implications for the development of synthetic chaperones for in vivo and in vitro applications.


Similar content being viewed by others
Abbreviations
- PDI:
-
Protein disulfide isomerase
- RNase (A):
-
Bovine pancreatic ribonuclease A
- DTT:
-
Dithiothreitol
- HPLC:
-
High performance liquid chromatography
References
Arolas JL, Aviles FX, Chang JY, Ventura S (2006) Trends Biochem Sci 31:292–301
Narayan M, Welker E, Wedemeyer WJ, Scheraga HA (2000) Acc Chem Res 33:737–820
Woycechowsky KJ, Raines RT (2000) Curr Opin Chem Bio l4:533–539 Review
Wedemeyer WJ, Welker E, Narayan M, Scheraga HA (2000) Biochemistry 39:4207–4216
Welker E, Narayan M, Wedemeyer WJ, Scheraga HA (2001) PNAS USA 98:2312–2316
Welker E, Wedemeyer WJ, Narayan M, Scheraga HA (2001) Biochemistry 40:9059–9064
Fewell SW, Travers KJ, Weissman JS, Brodsky JL (2001) Annu Rev Genet 35:149–191
Gilbert HF (1998) Methods Enzymol 290:26–50
Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H (2006) Cell 124:61–73 Erratum in: (2006) Cell 124:1085–1088
Tu BP, Weissman JS (2004) J Cell Biol 164:341–346
Wilkinson B, Gilbert HF (2004) Biochim Biophys Acta 1699:35–44
Chivers PT, Laboissiere MC, Raines RT (1996) EMBO J 15:2659–2667
Zheng J, Gilbert HF (2001) J Biol Chem 276:15747–15752
Shin HC, Scheraga HA (2000) J Mol Biol 4:995–1003
Weissman JS, Kim PS (1993) Nature 365:185–188
Gilbert HF, Kruzel ML, Lyles MM, Harper JW (1991) Protein Expr Purif 2:194–198
Rothwarf DM, Scheraga HA (1993) Biochemistry 32:2671–2679
Li YJ, Rothwarf DM, Scheraga HA (1995) Nat Struct Biol 2:489–494
Narayan M, Welker E, Scheraga HA (2001) J Am Chem Soc 123:2909–2910
Welker E, Narayan M, Volles MJ, Scheraga HA (1999) FEBS Lett. 460:477–479
Volles MJ, Xu X, Scheraga HA (1999) Biochemistry 38:7284–7293
Xu X, Rothwarf DM, Scheraga HA (1996) Biochemistry. 35:6406–6417
Saito K, Welker E, Scheraga HA (2001) Biochemistry 40:15002–15008
Iwaoka M, Wedemeyer WJ, Scheraga HA (1999) Biochemistry 38:2805–2815
Laity JH, Shimotakahara S, Scheraga HA (1993) Proc Natl Acad Sci USA 90:615–619
Hawkins HC, Freedman RB (1991) Biochem J 275:335–339
Kim JH, Johannes L, Goud B, Anthony C, Lingwood CA, Daneman R, Grinstein S (1998) PNAS USA 95:2997–3002
Kersteen EA, Raines RT (2003) Antioxid Redox Signal 5:413–424
Gough JD, Barrett EJ, Silva Y, Lees WJ (2006) J Biotechnol 125:39–47
Acknowledgements
This work has been possible through a start-up grant (UTEP) and discussions and assistance from Dr. Ervin Welker and Dr. John Xu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, YH., Narayan, M. pH Dependence of the Isomerase Activity of Protein Disulfide Isomerase: Insights into its Functional Relevance. Protein J 27, 181–185 (2008). https://doi.org/10.1007/s10930-007-9121-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-007-9121-0