Abstract
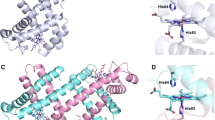
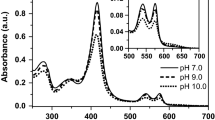
The interaction between human apohemoglobin A and CN-Mesohemin, a monomeric non-native heme derivative, was probed by Soret spectrophotometric titrations in 0.05 M potassium phosphate buffer, pH 7 at varied temperatures. Hypsochromic shifts in the absorbance maxima were observed at all temperatures below 10°C. First derivative spectroscopy of CN-Mesohemin titrations was used to provide further evidence of a spectral shift upon CN-Mesohemoglobin assembly. Findings of Soret Spectral shifts demonstrate a preference for the α chain heme site by CN-Mesohemin indicative of semi-α-hemoglobin intermediate formation. CN-Mesohemin, a derivative with peripheral 2,4 ethyl groups, does not possess the extended conjugation seen for native CN-Protohemin with its 2,4 vinyl groups. Indeed, reduced polarity of CN-Mesohemin over that of CN-Protohemin resulted in distinct temperature dependencies. Molecular visualization and protein-ligand interaction analysis targeted a functionally diverse residue unique to the α-chain. Tyrosine-42 (a polar/non-polar amino acid) appeared to play a prominent role in the assembly process.





Similar content being viewed by others
Reference
Ackers G. K., Holt J. M., Huang Y., Grinkova Y., Klinger A. L., Denisou I. (2000) Protein 4:23–43
Ascoli F., Fanelli M. R. R., Antonini E. (1981) Meth. Enzymol. 76:72–87
Boffi A., Zamparelli C., Verzili D., Ilari A., Chiancone E. (1997) Archives of Biochem. Biophys. 340:43–51
Brown S. B. (1980) An Introduction to Spectroscopy for Biochemists. Academic Press, A Subsidiary of Harcourt Brace Jovanovich, Publishers, London New York
Bunn H. F., Forget G. B. (1986) In: Dyson J. (ed) Hemoglobin: Molecular, Genetic and Clinical aspects. Saunders, Philadelphia, PA, pp 13–90
Fischer H., Orth H. (1937) Die Chemie des pyrroles, Pyrrolfarbstoffe. II. Erste Halfte. Leipzig Akcadem, Verlagsgesellschaft, pp. 372
Gibson Q. H. (1997) Archives of Biochem. Biophys. 339: 275–282
Gibson Q. H., Antonini E. (1963) J. Biol. Chem. 238: 1384–1388
Ho C. (1992) Adv. Pro. Chem. 43: 153–312
Ishimori K., Morishima I. (1988) Biochemistry 27: 4747–4753
Jennings T. M., McDonald M. J. (2002) Biochem. Biophys. Res. Comm. 293: 1354–1357
Joshi A. A., McDonald M. J. (1994) J. Biol. Chem.262: 5951–5956
Leutzinger Y., Beychok S. (1981) Proc. Natl. Acad. Sci. USA 78: 780–781
Park and Tame: To be published (PDB ID# 1IRD)
Perkampus H.H. (1992) UV-VIS Spectroscopy and its Applications. Springer Laboratory, Berlin Heidelberg, pp 88–94
Perutz M. F., Wilkinson A. J., Paoli M., Dodson G. G. (1998) Annu. Rev. Biophys. Biomol Struct. 27: 1–34
Smith K. M. (1975) Porphyrins and Metalloporphyrins. Elsevier Scientific Publishing Company, Amsterdam-Oxford-New York
Sobolev V., Sorokine A., Prilusky J., Abola E. E., Edelman M. (1999) Bioinformatics 15: 327–332
Stamatoyannopoulos G., Nienhuis A. W., Leader P., Majerus P. W. (1987) In: Dyson J. (ed) The Molecular Basis of Blood Disease, W.B. Saunders, Philadelphia PA, pp. 28–65
Vasudevan G., McDonald M. J. (1997) J. Biol. Chem. 272: 517–524
Vasudevan G., McDonald M. J. (2002) Curr. Prot. Pept. Sci. 3: 461–466
Vasudevan G., McDonald M. J. (2006) The Prot. J. 25: 45–56
Yamaguchi T., Pang J., Reddy K. S., Witkowska H. E., Surrey S., Adachi K. (1996) J. Biol. Chem. 271: 26677–26683
Zamperilli C., Boffi A., Verzili D., Chiancone E., Rousseau D.L., Takahashi S., Sugita Y., Yoneyama Y. (1971) J. Biol. Chem. 246:389–394
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fonseka, P.V., Vasudevan, G., Clarizia, LJ.A. et al. Temperature Dependent Soret Spectral Band Shifts Accompany Human CN-Mesohemoglobin Assembly. Protein J 26, 257–263 (2007). https://doi.org/10.1007/s10930-006-9067-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-9067-7