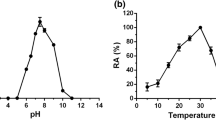
Trehalose, a naturally occurring osmolyte, is considered as a universal protein stabilizer. We investigated the effect of the disaccharides, trehalose and sucrose, on the thermal stability and conformation of bromelain. To our surprise, bromelain in the presence of 1 M trehalose/sucrose was destabilized under thermal stress. The average Tm values as determined by UV spectroscopy and CD spectropolarimetry decreased by 5° and 7°C for bromelain in 1 M sucrose or trehalose solutions, respectively. The enzyme was also found to inactivate faster at 60°C in the presence of these osmolytes. The tertiary and secondary structure of bromelain undergoes small changes in the presence of sucrose/trehalose. Studies on the binding of these osmolytes with the native and the heat denatured enzyme revealed that sucrose/trehalose lead to preferential hydration of the denatured bromelain as compared to the native one, hence stabilizing more the denatured conformation. This is perhaps the first report on the destabilization of a protein by trehalose.





Similar content being viewed by others

Abbreviations
- Bromelain:
-
pineapple stem bromelain
- FD:
-
fraction denatured
- CD:
-
circular dichroism
- T m :
-
mid-point of thermal transition
- SDS-PAGE:
-
sodium dodecyl sulphate-polyacylamide gel electrophoresis.
References
Arroya-Reyna A., Hernandez-Arana A. (1995). Biochem. Biophys. Acta 1248: 123–128
Arroya-Reyna A., Hernandez-Arana A., Arreguin-Espinosa R. (1994). Biochem. J. 300: 107–110
Brand L., Toptygin D.. (2000). Chem. Phys. Lett. 322: 492–502
Bulman A. L., Nelson H. C.. (2005). Proteins 58: 826–835
Carninci P., Nishiyana Y., Westover A., Itoh M., Nagaoka S., Sasaki N., Okazaki Y., Muramatsu M., Hayashizaki Y. (1998). Proc. Natl. Acad. Sci. U.S.A. 95: 520–524
D′Alfonso L., Collini M., Baldini G. (2003). Eur. J. Biochem. 270: 2497–2504
Demeester, J., Dekeyser, P. M., Samyn, N., Sierens, W., and Lauwers, A. (1997). In: Lauwer, A. and Scharpe, S. (eds.), Pharmaceutical Enzymes, Marcel Dekker, New York, pp. 343–385
Higashiyama T.. (2002). Pure Appl. Chem. 74: 1263–1269
Kaushik J. K., Bhat R.. (2003). J. Biol. Chem. 278: 26458–26465
Kreilgaard L., Frokjaer S., Flink J. M., Randolph T. W., Carpenter J. F.. (1998). Arch. Biochem. Biophys. 360: 121–134
Lin T. Y., Timasheff S. N.. (1996). Protein Sci. 5: 372–381
López-Diez E. C., Bone S.. (2004). Biochem. Biophys. Acta 1673: 139–148
Melo E. P., Faria T. Q., Martins L. O, Gonçalves A. M., Cabral J. M. (2001). Proteins 42: 542–552
Neumann D., Kohlbacher O., Lenhof H. P., Lehr C. M.. (2002). Eur. J. Biochem. 269: 1518–1524
Pawar S. A., Deshpande U. V.. (2000). Eur. J. Biochem. 267: 6331–6338
Ritonja A., Rowan A. D., Buttle D. J., Rowlings N. D., Turk V., Barett A. J.. (1989). FEBS Lett. 247: 419–424
Sola-Penna M., Ferreira-Pereira A., Lemos A. P., Meyer-Ferrandes J. R.. (1997). Eur. J. Biochem. 248: 24–29
Souillac P. O., Middaugh C. R., Rytting J. H.. (2002). Int. J. Pharm. 235: 207–218
Sun W. Q., Davidson P.. (1998). Biochem. Biophys. Acta 1425: 235–244
Vanhoof, G., and Cooremann, W. (1997). In: Lauwer, A. and Scharpe, S. (ed.), Pharmaceutical Enzymes, Marcel Dekker, New York, pp. 131–153
Von Seggern C. E., Cotter R. J.. (2004). J. Mass Spectrom. 39: 736–742
Wright W. W., Guffanti G. T., Vanderkooi J. M.. (2003). Biophys. J. 85: 1980–1995
Zancan P., Sola-Penna M.. (2005). Arch. Biochem. Biophys. 444: 52–60
Acknowledgements
Facilities provided by Aligarh Muslim University are gratefully acknowledged. The work was also supported by the department of Science and Technology, Government of India, under its FIST programme, and the University Grants Commission, India under its special assistance programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habib, S., Khan, M.A. & Younus, H. Thermal Destabilization of Stem Bromelain by Trehalose. Protein J 26, 117–124 (2007). https://doi.org/10.1007/s10930-006-9052-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-9052-1