Abstract
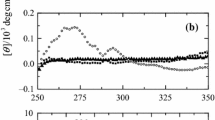
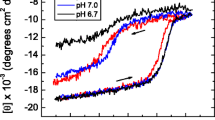
Structural and functional characteristics of jack bean urease (JBU), a hexameric enzyme having identical subunits, were investigated under neutral as well as acidic conditions by using CD, fluorescence, ANS binding and enzyme activity measurements. At low pH and low ionic strength, JBU exists in a partially unfolded state (UA-state), having predominantly β structure and no tertiary interactions along with a strong ANS binding. Addition of salts like NaCl, KCl and Na2SO4 to the UA-state induces refolding resulting in structural propensities similar to that of native hexamer. Moreover, at low concentrations, GuHCl behaves like an anion by inducing refolding of the UA-state. The anion-induced refolded state (IA-state) is more stable than UA-state and the stability is nearly equal to that of the native protein against chemical-induced and thermal denaturation. Overall, these observations support a model of protein folding for a multimeric protein where certain conformations (ensembles of substates) of low energy prevail and populated under non-native conditions with different stability.








Similar content being viewed by others
Abbreviations
- JBU:
-
jack bean urease
- GuHCl:
-
guanidine hydrochloride
- ANS:
-
8-anilino-1-naphthalenesulfonic acid
- CD:
-
circular dichroism
- UA :
-
acid-unfolded
- IA :
-
anion-refolded (anion-induced)
References
Arakwa T., Timasheff S. N. (1982). Biochemistry 21: 6545–6552
Baldwin R. L. (1995). J. Biomol. NMR 5: 103–109
Blakeley R. L., Webb E. C., Zerner B. (1969). Biochemistry 8: 1984–1990
Blattler D. P., Contaxis C. C., Reithel F. J. (1967). Nature 216: 274–275
Collins K. D., Washabaugh M. W. (1985). Q. Rev. Biophys. 18: 323–422
Edwin F., Jagannadham M. V. (1998). Biochem. Biophys. Res. Commun. 252: 654–660
Edwin F., Jagannadham M. V. (2000a). Arch. Biochem. Biophys. 381: 99–110
Fink A. L. (1995). Ann. Rev. Biophys. Biomol. Struct. 24: 495–522
Fink A. L., Calciano L. J., Goto Y., Nishimura M., Swedberg S. A. (1993). Protein Sci. 2:1155–1160
Fink A. L., Oberg K. A., Seshadri S. (1997). Fold. Des. 3: 1953–1960
Garel, J. R. (1992). In: Creighton, T. E. (ed.), Protein Folding, Freeman Co., New York, pp. 405–454
Gorin G., Blattler D. P., Thain D. E. (1969). Biochem. Biophys. Res. Commun. 36: 1045–1052
Goto Y., Fink A. L. (1989). Biochemistry 28: 945–952
Goto Y., Calciano L. J., Fink A. L. (1989). Proc. Natl. Acad. Sci. USA 87: 573–577
Goto Y., Calciano L. J., Fink A. L. (1990a). Proc. Natl. Acad. Sci. USA 87: 573–577
Goto Y., Takahashi N., Fink A. L. (1990b). Biochemistry 29: 3480–3488
Havel H. A., Kauffmann E.W., Plaisted W. M., Berms D. N. (1986). Biochemistry 25: 6533–6538
Hirai M., Kawai-Hirai R., Hirai T., Ukei T. (1993). Eur. J. Biochem. 215: 55–61
Ikeguchi M., Kuwazima K., Mitani M., Sugai S. (1986). Biochemistry 25: 6965–6972
Jabri E., Lee M. H., Hausinger R., Karplus P. A. (1992). J. Mol. Biol. 227: 934–937
Jaenicke R. (1987). Prog. Biophys. Mol. Biol. 49: 117–237
Jaenicke R. (1999). Prog. Biophys. Mol. Biol. 71: 155–241
Jagannadham M. V., Balasubramanian D. (1985). FEBS Lett. 188: 326–330
Jennings P. A., Wright P. E. (1993). Science 262: 892–896
Khurana R., Udgaonkar J. B. (1994). Biochemistry 33: 106–115
Kundu S., Sundd M., Jagannadham M. V. (1999). Biochem. Biophys. Res. Commun. 264: 635–642
Kuwajima K. (1989). Proteins: Struct. Funct. Genet. 6: 87–1
Levitte M., Chothia C. (1976) Nature 261: 552–558
Loh S. N., Kay M. S., Baldwin R. L. (1995). Proc. Natl. Acad. Sci. USA 92: 5446–5450
Mayr L. M., Schmid F. X. (1993). Biochemistry 32: 7994–7998
Mobley H. L. T., Hausinger R. P. (1989). Micro. Biol. Rev. 53: 85–108
Morjana N. A., McKeone B. J., Gilbert H. F. (1993). Proc. Natl. Acad. Sci. USA 90: 2107–2111
Morjana N. A., Tal R. (1998). Biotechnol. Appl. Biochem. 28: 7–17
Nolting B., Golbic R., Fersht A. R. (1995). Proc. Natl. Acad. Sci. USA 92: 10668–10672
Pace C. N., Aurents D. V., Thomson J. A. (1990). J. Biochem. 122: 83–89
Pace, C. N., Shirley, B. A., and Thomson, J. A. (1989). In: Creighton, T. E. (ed.), Protein Structure: A Practical Approach, vol. 5, IRL Press, Oxford, UK, pp. 311–330
Plaxco, K. W. and Dobson, C. M. (1996). Curr. Opin. Struct. Biol. 6: 630–636
Price, N. C. (1994). In: Pain, R. H. (ed.), Mechanisms of Protein Folding, IRL Press, New York, pp. 160–193
Privalov P. L. (1996). J. Mol. Biol. 258: 707–725
Ptitsyn, O. B. (1992). In: Creighton, T. E. (ed.), Protein Folding, Freeman Co., New York, pp. 243–300
Ptitsyn O. B. (1994). Protein Eng. 7: 593–596
Ptitsyn O. B (1995). Adv. Protein Chem. 47: 83–229
Radford S. E., Dobson C. M. (1995). Phil. Trans. R. Soc. Lond .B. 258: 707–725
Semisotnov G. V., Rodionova N. A., Razgulyaev O. I., Uversky V. N., Gripas A. F., Gilmanshin R. I. (1991). Biopolymers 31: 119–128
Sheridan L., Wilmot C. M., Cromie K. D., van der Loget P., Phillips S. E. (2002). Acta Crystallogr D Biol. Crystallogr. 58(pt 2): 374–376
Stryer L. (1965). J. Mol. Biol. 13: 482–495
Stryer L. (1968). Science 162: 526–540
Sumner J. B. (1926). J. Biol. Chem. 69: 435–441
Sundd M., Kundu S., Jagannadam M. V. (2002) J. Biochem. Mol. Biol. 35(2): 143–154
Tanford C. (1968). Adv. Protein Chem. 23: 121–282
Von Hipple P. H., Wong K. Y. (1965). J. Biol. Chem. 240: 3909–3923
Acknowledgment
We would like to thank Dr. Arvind M. Kayastha from School of Biotechnology, Faculty of Science, Banaras Hindu University for valuable suggestions. Authors duly acknowledge the Department of Biotechnology, Government of India for financial assistance and infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhowmick, R., Jagannadham, M.V. Multiple Intermediate Conformations of Jack Bean Urease at Low pH: Anion-induced Refolding. Protein J 25, 399–410 (2006). https://doi.org/10.1007/s10930-006-9026-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-9026-3