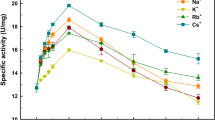
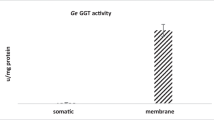
Abstract
A γ-glutamyltranspeptidase (GGT, E.C. 2.3.2.2) was isolated from a strain (A8) originating from Lake Bogoria (Kenya) and homologous with Bacillus pumilus. This GGT shows an optimal activity at pH 8.9 and 62°C. The enzyme is thermostable up to 43°C. The best reagent among the potential inhibitors was shown to be DON, which is an inhibitor highly specific for GGTs. Gly-Gly-Ala, Gly-Gly-Gly and Gly-Gly were identified as the best acceptors for the transpeptidation reactions catalyzed by the enzyme. The SDS-PAGE study revealed that the enzyme consists of two non-identical subunits (38,000 and 23,000). Only the large subunit was active when the enzyme was dissociated under denaturing conditions. The behavior of the native enzyme suggests that the active site of the large subunit is masked by the small subunit.




Similar content being viewed by others
Abbreviations
- DCI:
-
3,4-Dichloroisocoumarin
- DFP:
-
Di-isopropylfluorophosphate
- DON:
-
6-Diazo-5-oxo-l-norleucine
- E64:
-
Trans-epoxysuccinyl-l-leucylamido-(4-guanidino)-butane
- EDTA:
-
Ethylenediaminetetraacetic acid disodium salt
- EGTA:
-
Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- GGT:
-
Gamma-glutamyltranspeptidase
- PAGE:
-
Polyacrylamide gel electrophoresis
- PMSF:
-
Phenylmethanesulfonyl fluoride
- SDS:
-
Sodium dodecylsulfate
References
Abe K., Ito Y., Ohmachi T., Asada Y. (1997). Biosci. Biotechnol. Biochem. 61: 1621–1625
Castonguay R., Lherbet C., Keillor J. W. (2003) Biochemistry 42: 11504–11513
Dabonné S., Moallic C., Sine J.-P., Niamké S., Dion M., Colas B. (2005). Biochim. Biophys. Acta 1725: 136–143
Duckworth A. W., Grant W. D., Jones B. E., van Steenbergen R. (1996). FEMS Microbiol. Ecol. 19: 181–191
Gardell S. J., Tate S. S. (1979). J. Biol. Chem. 254: 4942–4945
Griffiths S. A., Manson M. M. (1989). Cancer Lett. 46: 69–74
Hashimoto W., Suzuki H., Nohara S., Tachi H., Yamamoto K., Kumagai H. (1995). J. Biochem. (Tokyo) 118: 1216–1223
Horiuchi S., Inoue M., Morino Y. (1978). Biochem. Biophys. Res. Commun. 80: 873–878
Horiuchi S., Inoue M., Morino Y. (1980). Eur. J. Biochem. 105: 93–102
Inoue M., Horiuchi S., Morino Y (1978). Biochem. Biophys. Res. Commun. 82: 1183–1188
Inoue M., Hiratake J., Suzuki H., Kumagai H., Sakata K. (2000). Biochemistry 39: 7764–7771
Ishiye M., Niwa M. (1992). FEMS Microbiol. Lett. 76: 235–241
Ishiye M., Yamashita M., Niwa M. (1993). Biotechnol. Prog. 9: 323–331
Kabanov A., Nametkin S., Chernov N., Klyachko N., Levashov A. (1991). FEBS Lett. 295: 73–76
Kimura K., Tran L.-S. P., Uchida I., Itoh Y. (2004) Microbiology 150: 4115–4123
Moallic, C. (2003) Thesis, Nantes, France
Ogawa Y., Hosoyama H., Hamano M., Motai H. (1991). Agric. Biol. Chem. 55: 2971–2977
Ogawa Y., Sugiura D., Motai H., Yuasa K., Tahara Y. (1997). Biosci. Biotechnol. Biochem. 61: 1596–1600
Papandrikopoulou A., Frey A., Gassen H. G. (1989). Eur. J. Biochem. 183: 693–698
Sakamuro D., Yamazoe M., Matsuda Y., Kangawa K., Taniguchi N., Matsuo H., Yoshikawa H., Ogasawara N. (1988). Gene 73: 1–9
Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985) Anal. Biochem. 150: 76–85
Suzuki H., Kumagai H., Tochikura T (1986a). J. Bacteriol. 168: 1325–1331
Suzuki H., Kumagai H., Tochikura T. (1986b). J. Bacteriol. 168: 1332–1335
Suzuki H., Kumagai H., Echigo T., Tochikura T. (1989). J. Bacteriol. 171: 5169–5172
Suzuki H., Hashimoto W., Kumagai H. (1999). J. Mol. Catal. B: Enzym. 6: 175–184
Suzuki H., Izuka S., Minami H., Miyakawa N., Ishihara S., Kumagai H. (2003) Appl. Environ. Microbiol. 69: 6399–6404
Tate S. S., Meister A. (1974). J. Biol. Chem. 249: 7593–7602
Tate S. S., Meister A. (1977). Proc. Natl. Acad. Sci. USA 74: 931–935
Tate S. S., Meister A. (1978). Proc. Natl. Acad. Sci. USA 75: 4806–4809
Xu K., Strauch M. A. (1996). J. Bacteriol. 178: 4319–4322
Acknowledgments
The authors would like to thank Dr F. Mulaa and Prof. D. Makawiti (University of Nairobi) for their help in harvesting sediment samples around Lake Bogoria, Dr M. Dion and Dr A. Defontaine (CNRS-UMR 6204, Nantes) for isolating and identifying the bacterial strains and J. C. Huet (INRA, Jouy-en-Josas) for determining the amino acid sequences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moallic, C., Dabonné, S., Colas, B. et al. Identification and Characterization of a Gamma-Glutamyl Transpeptidase from a Thermo-Alcalophile Strain of Bacillus pumilus . Protein J 25, 391–397 (2006). https://doi.org/10.1007/s10930-006-9025-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-9025-4