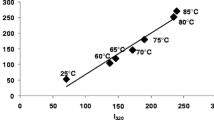
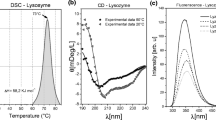
Binding sites for hydrophobic molecules on bovine β-lactoglobulin, and their susceptibility to temperature, were studied by using various spectroscopic probes. Binding of probes carrying a single fluorophore moiety, a single nitroxide moiety, or both moieties on the same molecule, was followed by EPR and fluorescence. The presence of a fatty acid side chain in the dual probes was found to be required for binding to β-lactoglobulin. Binding occurred only after the protein was heated at temperatures below the threshold for its irreversible denaturation. Binding became extremely tight and stable upon cooling of the protein–probe mixture. Comparison among the various probes suggests that multiple binding sites for hydrophobes are present in the native protein, and in the partially—and reversibly—modified form of β-lactoglobulin present in solution at neutral pH and subdenaturing temperatures. Thus, the specificity of hydrophobes binding to β-lactoglobulin may be modulated by simple physical treatment of the protein.
Similar content being viewed by others
Abbreviations
- amino-TEMPO:
-
1-Oxyl-4-amino-2,2,6,6-tetramethylpiperidine
- ANS:
-
1-anilinonaphthalene-8-sulfonic acid, sodium salt
- BLG:
-
bovine β-lactoglobulin
- CD:
-
circular dichroism
- DP1:
-
N-(1-Oxy1-2,2,6,6-tetramethylpiperidin-4-yl)-N-(carboxyundec-11-yl)-5-dimethylamino-1-naphtalene sulfonamide)
- DP2:
-
(1-Oxy1-2,2,6,6-tetramethylpiperidin-4-yl)-5-dimethylaminlo-1-naphtalene sulfonamide
- DSA:
-
5-DOXYL-stearic acid
- EPR:
-
electron paramagnetic resonance
References
A. Ahluwalia D. Rossi ParticleDe G. Giusto O. Chen V. Papper G.I. Likhtenstein (2002) Anal. Biochem. 305 121–134 Occurrence Handle10.1006/abio.2002.5601 Occurrence Handle1:CAS:528:DC%2BD38XktlGqu74%3D
A. Barbiroli F. Bonomi F. Faoro S. Iametti M. Iritri P. Rasmussen (2004) Ital. J. Biochem. 53 45
T. Biringhelli I Eberini M. Galliano A. Pedoto M. Perduca A. Sportiello E. Fontana H.L. Monaco E. Gianazza (2002) Biochemistry 41 14415–15422
S. Brownlow J. H. Morais Cabral R. Cooper D. R. Flower S. J. Yewdall I. Polikarpov A. C. T. North L.J. Sawyer (1997) Structure 5 481–495 Occurrence Handle10.1016/S0969-2126(97)00205-0 Occurrence Handle1:CAS:528:DyaK2sXjt1Sjt7Y%3D
I. M. Bystryak G. I. Likhtenshtein A. I. Kotelnikov H. O. Hankovsky K. Hideg (1986) Russian J. Phys. Chem. 60 1679–1683
S. Cairoli S. Iametti F. Bonomi (1994) J. Prot. Chem. 13 347–353 Occurrence Handle10.1007/BF01901568 Occurrence Handle1:CAS:528:DyaK2cXlsFWmu7o%3D
M. Collini L. D’Alfonso G. Baldini (2000) Protein Sci. 9 1968–1974 Occurrence Handle1:CAS:528:DC%2BD3cXosFKksbk%3D
D. Fessas S. Iametti A. Schiraldi F. Bonomi (2001) Eur. J. Biochem. 268 5439–5448 Occurrence Handle10.1046/j.0014-2956.2001.02484.x Occurrence Handle1:CAS:528:DC%2BD3MXnvFSgsr0%3D
R. Fugate P. Song (1980) Biochim. Biophys. Acta 625 28–42 Occurrence Handle1:CAS:528:DyaL3cXlvF2ltbY%3D
S. Futterman J. Heller (1972) J. Biol. Chem. 247 5168–5172 Occurrence Handle1:CAS:528:DyaE38XlsVyrsr8%3D
D. Hamada C. M. Dobson (2002) Protein Sci. 11 2417–2426 Occurrence Handle10.1110/ps.0217702 Occurrence Handle1:CAS:528:DC%2BD38XntlCrtrw%3D
Y.-H. Hong L. Creamer (2002) Food Sci. Biotech. 11 161–164 Occurrence Handle1:CAS:528:DC%2BD38Xmt1GmsLw%3D
S. Iametti F. Bonomi A. Fiocchi H. Frøkiær A. Gaiaschi C. Poiesi P. Rasmussen P. Restani P. Rovere (2003) J. Dairy. Res. 70 51–59 Occurrence Handle10.1017/S0022029902005678 Occurrence Handle1:CAS:528:DC%2BD3sXht1WgsLs%3D
S. Iametti S. Cairoli B. Gregori ParticleDe F. Bonomi (1995) J. Agr. Food Chem. 43 53–58 Occurrence Handle10.1021/jf00049a011 Occurrence Handle1:CAS:528:DyaK2MXjt1ClsL0%3D
S. Iametti B. Gregori ParticleDe G. Vecchio F. Bonomi (1996) Eur. J. Biochem. 237 106–112 Occurrence Handle10.1111/j.1432-1033.1996.0106n.x Occurrence Handle1:CAS:528:DyaK28Xitlyjur0%3D
S. Iametti P. Rasmussen H. Frøkiær P. Ferranti F. Addeo F. Bonomi (2002) Eur. J. Biochem. 269 1362–1372 Occurrence Handle10.1046/j.1432-1033.2002.02769.x Occurrence Handle1:CAS:528:DC%2BD38Xit1Cnsrg%3D
S. Iametti L. Scaglioni S. Mazzini G. Vecchio F. Bonomi (1998) J. Agric. Food Chem. 46 2159–2166 Occurrence Handle10.1021/jf980004b Occurrence Handle1:CAS:528:DyaK1cXjt1Ghtrc%3D
S. Iametti P. Transidico F. Bonomi G. Vecchio P. Pittia P. Rovere G. Dall’Aglio (1997) J. Agric. Food Chem. 45 23–29 Occurrence Handle10.1021/jf960330w Occurrence Handle1:CAS:528:DyaK2sXitVKisw%3D%3D
Jouenne, E., and Crouzet, J. (2000). J. Agr. Food Chem. 48: 5396–5400
P. Kolakowski E. Dumay J.-C. Cheftel (2001) Food Hydrocolloids 15 215–232 Occurrence Handle10.1016/S0268-005X(01)00017-0 Occurrence Handle1:CAS:528:DC%2BD3MXktVansL4%3D
G. Kontopidis C. Holt L. Sawyer (2002) J. Mol. Biol. 318 1043–1055 Occurrence Handle10.1016/S0022-2836(02)00017-7 Occurrence Handle1:CAS:528:DC%2BD38Xks1Krurc%3D
S. Kushibiki K. Hodate J. H. Kurisaki Y. Ueda A. Watanabe M. Shinoda (2001) J. Dairy Res. 68 579–586 Occurrence Handle10.1017/S0022029901005040 Occurrence Handle1:CAS:528:DC%2BD38XjtFKnu70%3D
J. R. Lakowicz (1999) Principles of Fluorescence Spectroscopy EditionNumber2 Kluwer Academic/Plenum Publishers New York
A. Laligant E. Dumay V. Casas C. Carmen L. Jean J. Cheftel (1991) J. Agric. Food Chem. 39 2147–2155 Occurrence Handle10.1021/jf00012a009 Occurrence Handle1:CAS:528:DyaK3MXmslygs7c%3D
D. Lange R. Kothari R. Patel S. Patel (1998) Biophys. Chem. 74 45–51 Occurrence Handle10.1016/S0301-4622(98)00164-1 Occurrence Handle1:CAS:528:DyaK1cXkvFOnu7g%3D
G. I. Likhtenshtein (1993) Biophysical Labeling Methods in Molecular Biology. Cambridge University Press Cambridge
M. Narayan L. J. Berliner (1997) Biochemistry 36 1906–1911 Occurrence Handle10.1021/bi9621526 Occurrence Handle1:CAS:528:DyaK2sXhvFGgtLg%3D
M. Z. Papiz L. J. Sawyer E. E. Eliopoulos A. C. T. North J. B. Findlay R. Sivaprasadarao T. A. Jones M. E. Newcomer P. J. Kraulis (1986) Nature 324 383–385 Occurrence Handle10.1038/324383a0 Occurrence Handle1:CAS:528:DyaL2sXksF2ktw%3D%3D
Ptitsyn O.B. (1992). In: Creighton, T. E. (ed.), Protein Folding. Freeman, New York, pp. 243–300
Ptitsyn O.B., and Semisotnov G.V. (1991). In: Nall, B. T., and Dill, K. A. (eds), Conformations and Forces in Protein Folding. AAAS, Washington, DC, pp. 155–168
L. Ragona F. Fogolari L. Zetta D. M. Perez P. Puyol K. G. Kruif ParticleDe F. Lohr H. Ruterjans H. Molinari (2000) Protein Sci. 9 1347–1356 Occurrence Handle1:CAS:528:DC%2BD3cXltl2hsLo%3D Occurrence Handle10.1110/ps.9.7.1347
K. Robillard A. Wishnia (1972) Biochemistry 11 3835–3840 Occurrence Handle10.1021/bi00771a001 Occurrence Handle1:CAS:528:DyaE38XlsFOiur0%3D
S. P. F. M. Roefs K. G. Kruif ParticleDe (1994) Eur. J. Biochem. 226 883–889 Occurrence Handle10.1111/j.1432-1033.1994.00883.x Occurrence Handle1:CAS:528:DyaK2cXmvFGgurw%3D
R. A. Sayle E. J. Milner-White (1995) Trends Biochem. Sc. 20 374–376 Occurrence Handle10.1016/S0968-0004(00)89080-5 Occurrence Handle1:CAS:528:DyaK2MXotF2rsLg%3D
G. V. Semisotnov N. Rodionova O. Razgulyaev V. Uversy A. Gripas R. Gilmanshin (1991) Biopolymers 31 119–128 Occurrence Handle10.1002/bip.360310111 Occurrence Handle1:CAS:528:DyaK3MXhvVWmurY%3D
A. Spector K. John J. Fletcher (1969) J. Lipid Res. 10 56–67 Occurrence Handle1:CAS:528:DyaF1MXmsVCntQ%3D%3D
N. V. Strashnikova N. Medvedeva G.I. Likhtenshtein (2001) J. Biochem. Biophys. Meth. 48 43–60 Occurrence Handle10.1016/S0165-022X(00)00144-5 Occurrence Handle1:CAS:528:DC%2BD3MXitF2rs70%3D
L. Stryer H. O. Griffith (1965) Proc. Natl. Acad. Sci. USA 54 1785–1791 Occurrence Handle1:CAS:528:DyaF28XntVerug%3D%3D
A. Touati C. Creuzenet J. M. Chobert E. Dufour T. J. Haertle (1992) Protein Chem. 11 613–621 Occurrence Handle10.1007/BF01024961 Occurrence Handle1:CAS:528:DyaK3sXjsVOhtQ%3D%3D
S. Y. Wu M. D. Perez P. Puyol L. J. Sawyer (1999) Biol. Chem. 274 170–174 Occurrence Handle10.1074/jbc.274.1.170 Occurrence Handle1:CAS:528:DyaK1MXjvVWmuw%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lozinsky, E., Iametti, S., Barbiroli, A. et al. Structural Features of Transiently Modified Beta-Lactoglobulin Relevant to the Stable Binding of Large Hydrophobic Molecules. Protein J 25, 1–15 (2006). https://doi.org/10.1007/s10930-006-0016-2
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10930-006-0016-2