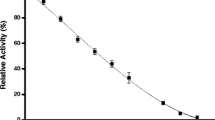
The effects of guanidinium chloride (GuHCl) on the activity of Penaeus vannamei β-N-acetyl-d-glucosaminidase (NAGase) have been studied. The results show that GuHCl, at appropriate concentrations, can lead to reversible inactivation of the enzyme, and the IC50 is estimated to be 0.6 M. Changes of activity and conformation of the enzyme in different concentrations of GuHCl have been studied by measuring the fluorescence spectra and its relative activity after denaturation. The fluorescence intensity of the enzyme decreases distinctly with increasing GuHCl concentrations, and the emission peaks appear red-shifted (from 339.4 to 360 nm). Changes in the conformation and catalytic activity of the enzyme are compared. The extent of inactivation is greater than that of conformational changes, indicating that the active site of the enzyme is more flexible than the whole enzyme molecule. The kinetics of inactivation has been studied using the kinetic method of the substrate reaction. The rate constants of inactivation have been determined. The value of k+0 is larger than that of k ′+0 which suggests that the enzyme is protected by substrate to a certain extent during guanidine denaturation.
Similar content being viewed by others
Abbreviations
- NAGase:
-
β-N-acetyl-d-glucosaminidase
- NAG:
-
N-acetylglucosamine
- GuHCl:
-
guanidinium chloride
- pNP-NAG:
-
p-nitrophenyl-N-acetyl-β-d-glucosaminide
- pNP:
-
p-nitrophenol
- PAGE:
-
polyacrylamide gel electrophoresis
- SDS-PAGE:
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- NaAc-HAc:
-
sodium acetate–acetate buffer
- NaOH:
-
sodium hydroxide
- IC50:
-
the inactivator concentration leading to 50% of enzyme activity lost.
References
R. D. Cannon K. Nimi H. F. Jenkinson M. G. Shepherd (1994) J. Bacteriol. 176 2640–2647 Occurrence Handle1:CAS:528:DyaK2cXkslCrtL4%3D
Q. X. Chen W. Zhang W. Z. Zheng Z. Zhang S. X. Yan T. Zhang H. M. Zhou (1996a) J. Protein Chem. 15 359–365 Occurrence Handle1:CAS:528:DyaK28Xkt1Gktrg%3D
Q. X. Chen W. Zhang W. Z. Zheng H. Zhao S. H. Yan H. R. Wang H. M. Zhou (1996b) J. Protein Chem. 15 345–350 Occurrence Handle1:CAS:528:DyaK28Xkt1Gktro%3D
Q. X. Chen Z. Zhang H. Huang F. K. Zhao G. J. Xu (2003) Int. J. Biochem. Cell Biol. 35 1227–1233 Occurrence Handle10.1016/S1357-2725(02)00266-2 Occurrence Handle1:CAS:528:DC%2BD3sXjvFektbg%3D
P. J. Espie J. C. Roff (1995) Physiol. Zool. 68 727–748 Occurrence Handle1:CAS:528:DyaK2MXovFensbg%3D
B. Funke K. D. Spindler (1987) NoChapterTitle C. Decleir (Eds) et al. Artemia Research and its Application Universa Press Wetteren 67–78
D. Koga C. Shimazaki K. Yamamoto K. Inoue S. Kimura A. Ide (1987) Agric. Biol. Chem. 51 1679–1681 Occurrence Handle1:CAS:528:DyaL2sXltFWlsrk%3D
M. Kono T. Matsui C. Shimizu D. Koga (1990) Agric. Biol. Chem. 54 2145–2147 Occurrence Handle1:CAS:528:DyaK3cXlvVKqu7Y%3D
O. H. Lowry N. J. Rosebrough A. L. Farr R. J. Randall (1951) J. Biol. Chem. 193 265–269 Occurrence Handle1:CAS:528:DyaG38XhsVyrsw%3D%3D
K. R. Lynn (1990) Comp. Biochem. Physiol. 96 IssueIDB 761–766
G. Peters R. Saborowski F. Buchholz R. Mentlein (1999) Mar. Biol. 134 697–703 Occurrence Handle10.1007/s002270050585 Occurrence Handle1:CAS:528:DyaK1MXmsFynu7Y%3D
C. L. Tsou (1993) Science 268 380–381
C. L. Tsou (1988) Adv. Enzymol. Related Areas Mol. Biol. 61 381–436 Occurrence Handle1:CAS:528:DyaL1MXhtlSrtbY%3D
H. R. Wang X. C. Wang T. Zhang H. M. Zhou (1995) Sci. China 38 IssueIDB 328–335 Occurrence Handle1:CAS:528:DyaK2MXlvVKrsrk%3D
J. Xiao S. J. Liang C. L. Tsou (1993) Biochim. Biophys. Acta. 1164 54–60 Occurrence Handle1:CAS:528:DyaK3sXmt1Sgtbg%3D
X. L. Xie Q. X. Chen (2004) Biochemistry (Moscow) 69 1675–1682 Occurrence Handle10.1007/s10541-005-0082-7
X. L. Xie Q. X. Chen J. C. Lin Y. Wang (2004) Mar. Biol. 146 143–148 Occurrence Handle10.1007/s00227-004-1425-4 Occurrence Handle1:CAS:528:DC%2BD2cXhtVals7rP
K. C. Zen H. K. Choi N. Krishnamachary S. Muthukrishnan K. J. Kramer (1996) Insect Biochem. Molec. Biol. 26 435–444 Occurrence Handle10.1016/0965-1748(95)00111-5 Occurrence Handle1:CAS:528:DyaK28XktVyksbo%3D
E. Zou M. Fingerman (1999) Mar. Biol. 133 97–101 Occurrence Handle10.1007/s002270050447 Occurrence Handle1:CAS:528:DyaK1MXhsFCns70%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, XL., Chen, QX., Gong, M. et al. Inactivation Kinetics of Guanidinium Chloride on Penaeus vannameiβ - N-Acetyl-D-Glucosaminidase and the Relationship of Enzyme Activity and its Conformation. Protein J 24, 267–273 (2005). https://doi.org/10.1007/s10930-005-6747-7
Issue Date:
DOI: https://doi.org/10.1007/s10930-005-6747-7