Abstract
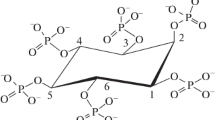
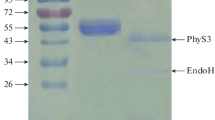
A periplasmatic phytate-degrading enzyme from Pantoea agglomerans isolated from soil was purified about 470-fold to apparent homogeneity with a recovery of 16% referred to the phytate-degrading activity in the crude extract. It behaved as a monomeric protein with a molecular mass of about 42 kDa. The purified enzyme exhibited a single pH optimum at 4.5. Optimum temperature for the degradation of phytate was 60°C. The kinetic parameters for the hydrolysis of sodium phytate were determined to be KM = 0.34 mmol/l and kcat = 21 s-1 at pH 4.5 and 37°C. The enzyme exhibited a narrow substrate selectivity. Only phytate and glucose-1-phosphate were identified as good substrates. Since this Pantoea enzyme has a strong preference for glucose-1-phosphate over phytate, under physiological conditions glucose-1-phosphate is its most likely substrate. The maximum amount of phosphate released from phytate by the purified enzyme suggests myo-inositol pentakisphosphate as the final product of enzymatic phytate degradation.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greiner, R. Purification and Properties of a Phytate-degrading Enzyme from Pantoea agglomerans. Protein J 23, 567–576 (2004). https://doi.org/10.1007/s10930-004-7883-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10930-004-7883-1