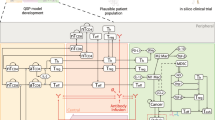
Abstract
Non-Small Cell Lung Cancer (NSCLC) remains one of the main causes of cancer death worldwide. In the urge of finding an effective approach to treat cancer, enormous therapeutic targets and treatment combinations are explored in clinical studies, which are not only costly, suffer from a shortage of participants, but also unable to explore all prospective therapeutic solutions. Within the evolving therapeutic landscape, the combined use of radiotherapy (RT) and checkpoint inhibitors (ICIs) emerged as a promising avenue. Exploiting the power of quantitative system pharmacology (QSP), we undertook a study to anticipate the therapeutic outcomes of these interventions, aiming to address the limitations of clinical trials. After enhancing a pre-existing QSP platform and accurately replicating clinical data outcomes, we conducted an in-depth study, examining different treatment protocols with nivolumab and RT, both as monotherapy and in combination, by assessing their efficacy through clinical endpoints, namely time to progression (TTP) and duration of response (DOR). As result, the synergy of combined protocols showcased enhanced TTP and extended DOR, suggesting dual advantages of extended response and slowed disease progression with certain combined regimens. Through the lens of QSP modeling, our findings highlight the potential to fine-tune combination therapies for NSCLC, thereby providing pivotal insights for tailoring patient-centric therapeutic interventions.





Similar content being viewed by others
References
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer Immunoediting: integrating immunity’s roles in Cancer suppression and Promotion. Science 331:1565–1570. https://doi.org/10.1126/science.1203486
Upadhaya S, Neftelinov ST, Hodge J, Campbell J (2022) Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov 21:482–483. https://doi.org/10.1038/d41573-022-00030-4
Chelliah V, Lazarou G, Bhatnagar S et al (2021) Quantitative systems Pharmacology Approaches for Immuno-Oncology: adding virtual patients to the Development paradigm. Clin Pharmacol Ther 109:605–618. https://doi.org/10.1002/cpt.1987
Prasad V, Mailankody S (2017) Research and development spending to bring a single Cancer drug to market and revenues after approval. JAMA Intern Med 177:1569. https://doi.org/10.1001/jamainternmed.2017.3601
Bradshaw EL, Spilker ME, Zang R et al (2019) Applications of quantitative systems Pharmacology in Model-Informed Drug Discovery: perspective on Impact and opportunities. CPT Pharmacometrics Syst Pharmacol 8:777–791. https://doi.org/10.1002/psp4.12463
Sové RJ, Jafarnejad M, Zhao C et al (2020) QSP-IO: a quantitative systems Pharmacology Toolbox for mechanistic Multiscale modeling for Immuno‐Oncology Applications. Clin Pharmacol Ther 9:484–497. https://doi.org/10.1002/psp4.12546
Kosinsky Y, Dovedi SJ, Peskov K et al (2018) Radiation and PD-(L)1 treatment combinations: immune response and dose optimization via a predictive systems model. j Immunotherapy cancer 6:17. https://doi.org/10.1186/s40425-018-0327-9
Balti A, Zugaj D, Fenneteau F et al (2021) Dynamical systems analysis as an additional tool to inform treatment outcomes: the case study of a quantitative systems pharmacology model of immuno-oncology. Chaos 31:023124. https://doi.org/10.1063/5.0022238
Zugaj D, Fenneteau F, Tremblay P-O, Nekka F (2024) Dynamical behavior-based approach for the evaluation of treatment efficacy: The case of immuno-oncology. Chaos Interdiscip J Nonlinear Sci 34:013142. https://doi.org/10.1063/5.0170329
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355. https://doi.org/10.1126/science.aar4060
Postmus PE, Kerr KM, Oudkerk M et al (2017) Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 28:iv1–iv21. https://doi.org/10.1093/annonc/mdx222
Shaverdian N, Lisberg AE, Bornazyan K et al (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895–903. https://doi.org/10.1016/S1470-2045(17)30380-7
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer. J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Molina JR, Yang P, Cassivi SD et al (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc. https://doi.org/10.4065/83.5.584
Gettinger S, Rizvi NA, Chow LQ et al (2016) Nivolumab Monotherapy for First-Line treatment of Advanced non–small-cell Lung Cancer. JCO 34:2980–2987. https://doi.org/10.1200/JCO.2016.66.9929
Topalian SL, Hodi FS, Brahmer JR et al (2019) Five-year survival and correlates among patients with Advanced Melanoma, Renal Cell Carcinoma, or non–small cell lung Cancer treated with Nivolumab. JAMA Oncol 5:1411. https://doi.org/10.1001/jamaoncol.2019.2187
Kim H, Chung J-H (2019) PD-L1 testing in non-small cell lung Cancer: past, Present, and Future. J Pathol Transl Med 53:199–206. https://doi.org/10.4132/jptm.2019.04.24
Grigg C, Rizvi NA (2016) PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction? j Immunotherapy cancer 4:48. https://doi.org/10.1186/s40425-016-0153-x
Chajon E, Castelli J, Marsiglia H, De Crevoisier R (2017) The synergistic effect of radiotherapy and immunotherapy: a promising but not simple partnership. Crit Rev Oncol/Hematol 111:124–132. https://doi.org/10.1016/j.critrevonc.2017.01.017
Yang H, Jin T, Li M et al (2019) Synergistic effect of immunotherapy and radiotherapy in non-small cell lung cancer: current clinical trials and prospective challenges. Precision Clin Med 2:57–70. https://doi.org/10.1093/pcmedi/pbz004
Jafarnejad M, Gong C, Gabrielson E et al (2019) A computational model of neoadjuvant PD-1 inhibition in Non-small Cell Lung Cancer. AAPS J 21:79. https://doi.org/10.1208/s12248-019-0350-x
Benzekry S, Lamont C, Beheshti A et al (2014) Classical Mathematical models for description and prediction of experimental Tumor Growth. PLoS Comput Biol 10:e1003800. https://doi.org/10.1371/journal.pcbi.1003800
Dhar M, Bhattacharya P (2018) Comparison of the logistic and the Gompertz curve under different constraints. J Stat Manage Syst 21:1189–1210. https://doi.org/10.1080/09720510.2018.1488414
Vaghi C, Rodallec A, Fanciullino R et al (2020) Population modeling of tumor growth curves and the reduced Gompertz model improve prediction of the age of experimental tumors. PLoS Comput Biol 16:e1007178. https://doi.org/10.1371/journal.pcbi.1007178
Suleiman AA, Nogova L, Fuhr U (2013) Modeling NSCLC progression: recent advances and opportunities available. AAPS J 15:542–550. https://doi.org/10.1208/s12248-013-9461-y
Geng C, Paganetti H, Grassberger C (2017) Prediction of treatment response for combined chemo- and Radiation Therapy for Non-small Cell Lung Cancer patients using a Bio-mathematical Model. Sci Rep 7:13542. https://doi.org/10.1038/s41598-017-13646-z
Walle T, Martinez Monge R, Cerwenka A et al (2018) Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol 10:175883401774257. https://doi.org/10.1177/1758834017742575
Brenner DJ, Hlatky LR, Hahnfeldt PJ et al (1998) The Linear-Quadratic Model and Most Other Common Radiobiological models result in similar predictions of Time-Dose relationships. Radiat Res 150:83. https://doi.org/10.2307/3579648
Sachs RK, Hlatky LR, Hahnfeldt P (2001) Simple ODE models of tumor growth and anti-angiogenic or radiation treatment. Math Comput Model 33:1297–1305. https://doi.org/10.1016/S0895-7177(00)00316-2
Wang H, Sové RJ, Jafarnejad M et al (2020) Conducting a virtual clinical trial in HER2-Negative breast Cancer using a quantitative systems Pharmacology Model with an epigenetic modulator and Immune Checkpoint inhibitors. Front Bioeng Biotechnol 8:141. https://doi.org/10.3389/fbioe.2020.00141
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Ye H, Pang H, Shi X et al (2021) Nivolumab and Hypofractionated Radiotherapy in patients with Advanced Lung Cancer: ABSCOPAL-1 clinical trial. Front Oncol 11:657024. https://doi.org/10.3389/fonc.2021.657024
Barbee MS, Ogunniyi A, Horvat TZ, Dang T-O (2015) Current status and future directions of the Immune checkpoint inhibitors Ipilimumab, Pembrolizumab, and Nivolumab in oncology. Ann Pharmacother 49:907–937
Zhang Z, Liu X, Chen D, Yu J (2022) Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Sig Transduct Target Ther 7:258. https://doi.org/10.1038/s41392-022-01102-y
Rodrigues G, Choy H, Bradley J et al (2015) Definitive radiation therapy in locally advanced non-small cell lung cancer: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based clinical practice guideline. Practical Radiation Oncol 5:141–148. https://doi.org/10.1016/j.prro.2015.02.012
Ko EC, Raben D, Formenti SC (2018) The Integration of Radiotherapy with Immunotherapy for the treatment of non–small cell Lung Cancer. Clin Cancer Res 24:5792–5806. https://doi.org/10.1158/1078-0432.CCR-17-3620
Serritella AV, Shenoy NK (2023) Nivolumab Plus Ipilimumab vs Nivolumab alone in Advanced Cancers Other Than Melanoma: a Meta-analysis. JAMA Oncol 9:1441. https://doi.org/10.1001/jamaoncol.2023.3295
Kim Y-J, Oremus M, Chen HH et al (2020) Real-world effectiveness of nivolumab in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Future Oncol 16:2045–2058. https://doi.org/10.2217/fon-2020-0248
Prise KM, Schettino G, Folkard M, Held KD (2005) New insights on cell death from radiation exposure. Lancet Oncol 6:520–528. https://doi.org/10.1016/S1470-2045(05)70246-1
Wang H, Ma H, Sové RJ et al (2021) Quantitative systems pharmacology model predictions for efficacy of atezolizumab and nab-paclitaxel in triple-negative breast cancer. J Immunother Cancer 9:e002100. https://doi.org/10.1136/jitc-2020-002100
Kang J, Demaria S, Formenti S (2016) Current clinical trials testing the combination of immunotherapy with radiotherapy. j Immunotherapy cancer 4:51. https://doi.org/10.1186/s40425-016-0156-7
Antonia SJ, Villegas A, Daniel D et al (2018) Overall survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 379:2342–2350. https://doi.org/10.1056/NEJMoa1809697
Chang JY, Lin SH, Dong W et al (2023) Stereotactic ablative radiotherapy with or without immunotherapy for early-stage or isolated lung parenchymal recurrent node-negative non-small-cell lung cancer: an open-label, randomised, phase 2 trial. The Lancet. https://doi.org/10.1016/S0140-6736(23)01384-3
Yang J, Yue J-B, Liu J, Yu J-M (2014) Repopulation of tumor cells during fractionated radiotherapy and detection methods (review). Oncol Lett 7:1755–1760. https://doi.org/10.3892/ol.2014.1990
Williamson CW, Sherer MV, Zamarin D et al (2021) Immunotherapy and radiation therapy sequencing: state of the data on timing, efficacy, and safety. Cancer 127:1553–1567. https://doi.org/10.1002/cncr.33424
McCaw ZR, Tian L, Wei L-J (2020) Appropriate analysis of duration of Response Data in Cancer trials. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.4657. 6:1978
Rieger TR, Allen RJ, Bystricky L et al (2018) Improving the generation and selection of virtual populations in quantitative systems pharmacology models. Prog Biophys Mol Biol 139:15–22. https://doi.org/10.1016/j.pbiomolbio.2018.06.002
Allen R, Rieger T, Musante C (2016) Efficient generation and selection of virtual populations in quantitative systems Pharmacology models. CPT Pharmacometrics Syst Pharmacol 5:140–146. https://doi.org/10.1002/psp4.12063
Sinisi S, Alimguzhin V, Mancini T et al (2021) Complete populations of virtual patients for in silico clinical trials. Bioinformatics 36:5465–5472. https://doi.org/10.1093/bioinformatics/btaa1026
Surendran A, Le Sauteur-Robitaille J, Kleimeier D et al (2023) Approaches to Generating virtual patient cohorts with applications in Oncology. In: Cesario A, D’Oria M, Auffray C, Scambia G (eds) Personalized Medicine meets Artificial Intelligence. Springer International Publishing, Cham, pp 97–119
Wang H, Arulraj T, Kimko H, Popel AS (2023) Generating immunogenomic data-guided virtual patients using a QSP model to predict response of advanced NSCLC to PD-L1 inhibition. npj Precis Onc 7:55. https://doi.org/10.1038/s41698-023-00405-9
Milberg O, Gong C, Jafarnejad M et al (2019) A QSP Model for Predicting clinical responses to Monotherapy, Combination and Sequential Therapy following CTLA-4, PD-1, and PD-L1 checkpoint blockade. Sci Rep 9:11286. https://doi.org/10.1038/s41598-019-47802-4
Weidhaas J, Marco N, Scheffler AW et al (2022) Germline biomarkers predict toxicity to anti-PD1/PDL1 checkpoint therapy. J Immunother Cancer 10:e003625. https://doi.org/10.1136/jitc-2021-003625
Acknowledgements
M.S. received the Bourse du centenaire from the Faculté de Pharmacie, Université de Montréal. Support was also provided by NSERC (Collaborative Research and Development Grants), in partnership with Syneos Health and Pfizer; FRQNT-Projet d’équipe, and from Prompt.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.S., D.Z., P.-O.T., F.N.; Methodology: M.S., H.C., D.Z., F.F., F.N.; Code development and simulations: M.S., D.Z.; Investigation: M.S., H.C., D.Z.; Data acquisition: M.S., K.-E.I., H.C.; Funding acquisition: F.N., P.-O.T.; Writing—original draft: M.S.; Writing—review & editing: M.S., F.F., D.Z., F,N.; Supervision: F.N., F.F., D.Z.. All authors reviewed and authorized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schirru, M., Charef, H., Ismaili, KE. et al. Predicting efficacy assessment of combined treatment of radiotherapy and nivolumab for NSCLC patients through virtual clinical trials using QSP modeling. J Pharmacokinet Pharmacodyn (2024). https://doi.org/10.1007/s10928-024-09903-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10928-024-09903-0