Abstract
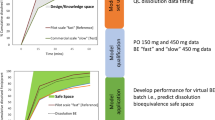
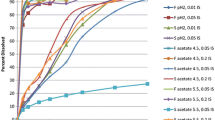
We propose a Bayesian population modeling and virtual bioequivalence assessment approach to establishing dissolution specifications for oral dosage forms. A generalizable semi-physiologically based pharmacokinetic absorption model with six gut segments and liver, connected to a two-compartment model of systemic disposition for bupropion hydrochloride oral dosage forms was developed. Prior information on model parameters for gut physiology, bupropion physicochemical properties, and drug product properties were obtained from the literature. The release of bupropion hydrochloride from immediate-, sustained- and extended-release oral dosage forms was described by a Weibull function. In vitro dissolution data were used to assign priors to the in vivo release properties of the three bupropion formulations. We applied global sensitivity analysis to identify the influential parameters for plasma bupropion concentrations and calibrated them. To quantify inter- and intra-individual variability, plasma concentration profiles in healthy volunteers that received the three dosage forms, each at two doses, were used. The calibrated model was in good agreement with both in vitro dissolution and in vivo exposure data. Markov Chain Monte Carlo samples from the joint posterior parameter distribution were used to simulate virtual crossover clinical trials for each formulation with distinct drug dissolution profiles. For each trial, an allowable range of dissolution parameters (“safe space”) in which bioequivalence can be anticipated was established. These findings can be used to assure consistent product performance throughout the drug product life-cycle and to support manufacturing changes. Our framework provides a comprehensive approach to support decision-making in drug product development.









Similar content being viewed by others
References
U.S. Food and Drug Administration (2017) CFR-code of federal regulations title 21
U.S. Food and Drug Administration (2017) Product-specific guidances for generic drug development
Khan S, Berendt R, Ellison C, Ciavarella A, Asafu-Adjaye E, Khan M, Faustino P (2016) Bupropion hydrochloride. Profiles Drug Subst Excip Related s 41:1–30. https://doi.org/10.1016/bs.podrm.2015.12.001
Connarn JN, Zhang X, Babiskin A, Sun D (2015) Metabolism of bupropion by carbonyl reductases in liver and intestine. Drug Metab Dispos 43:1019–1027. https://doi.org/10.1124/dmd.115.063107
Sager JE, Price LS, Isoherranen N (2016) Stereoselective metabolism of bupropion to OH-bupropion, threohydrobupropion, erythrohydrobupropion, and 4′-OH-bupropion in vitro. Drug Metab Dispos 44:1709–1719. https://doi.org/10.1124/dmd.116.072363
Lionberger R, Uhl K (2019) Generic drugs: expanding possibilities for clinical pharmacology. Clin Pharmacol Ther 105:278–281. https://doi.org/10.1002/cpt.1320
Zhao L, Kim M-J, Zhang L, Lionberger R (2019) Generating model integrated evidence for generic drug development and assessment. Clin Pharmacol Ther 105:338–349. https://doi.org/10.1002/cpt.1282
Al-Tabakha MM, Alomar MJ (2020) In vitro dissolution and in silico modeling shortcuts in bioequivalence testing. Pharmaceutics 12:45. https://doi.org/10.3390/pharmaceutics12010045
Miao L, Mousa YM, Zhao L, Raines K, Seo P, Wu F (2020) Using a physiologically based pharmacokinetic absorption model to establish dissolution bioequivalence safe space for oseltamivir in adult and pediatric populations. AAPS J 22:1–10. https://doi.org/10.1208/s12248-020-00493-6
Bonate PL (2011) Pharmacokinetic-pharmacodynamic modeling and simulation. Springer
Zhao P, Rowland M, Huang S-M (2012) Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther 92:17–20. https://doi.org/10.1038/clpt.2012.68
Grimstein M, Yang Y, Zhang X, Grillo J, Huang S-M, Zineh I, Wang Y (2019) Physiologically based pharmacokinetic modeling in regulatory science: an update from the US food and drug administration’s office of clinical pharmacology. J Pharm Sci 108:21–25. https://doi.org/10.1016/j.xphs.2018.10.033
U.S. Food and Drug Administration (2018) Physiologically based pharmacokinetic analyses—format and content. Guidance for industry
European Medicine Agency (2018) Guideline on the reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation
Suarez-Sharp S, Cohen M, Kesisoglou F, Abend A, Marroum P, Delvadia P, Kotzagiorgis E, Li M, Nordmark A, Bandi N, et al (2018) Applications of clinically relevant dissolution testing: Workshop summary report
Heimbach T, Suarez-Sharp S, Kakhi M, Holmstock N, Olivares-Morales A, Pepin X, Sjögren E, Tsakalozou E, Seo P, Li M, et al (2019) Dissolution and translational modeling strategies toward establishing an in vitro-in vivo link—a workshop summary report
Kesisoglou F, Mitra A (2015) Application of absorption modeling in rational design of drug product under quality-by-design paradigm. AAPS J 17:1224–1236. https://doi.org/10.1208/s12248-015-9781-1
Butler J, Hens B, Vertzoni M, Brouwers J, Berben P, Dressman J, Andreas CJ, Schaefer KJ, Mann J, McAllister M et al (2019) In vitro models for the prediction of in vivo performance of oral dosage forms: recent progress from partnership through the IMI OrBiTo collaboration. Eur J Pharm Biopharm 136:70–83. https://doi.org/10.1016/j.ejpb.2018.12.010
Pepin XJ, Parrott N, Dressman J, Delvadia P, Mitra A, Zhang X, Babiskin A, Kolhatkar V, Suarez-Sharp S (2020) Current state and future expectations of translational modeling strategies to support drug product development, manufacturing changes and controls: a workshop summary report. J Pharm Sci. https://doi.org/10.1016/j.xphs.2020.04.021
Mould D, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development—part 2: introduction to pharmacokinetic modeling methods. CPT 2:1–14. https://doi.org/10.1038/psp.2013.14
Jamei M (2020) Where do PBPK models stand in pharmacometrics and systems pharmacology? CPT. https://doi.org/10.1002/psp4.12493
Gelman A, Bois FY, Jiang J (1996) Physiological pharmacokinetic analysis using population modeling and informative prior distributions. J Am Stat Assoc 91:1400–1412. https://doi.org/10.1080/01621459.1996.10476708
Zurlinden TJ, Heard K, Reisfeld B (2016) A novel approach for estimating ingested dose associated with paracetamol overdose. Br J Clin Pharmacol 81:634–645. https://doi.org/10.1111/bcp.12796
Lawrence XY, Amidon GL (1999) A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm 186:119–125. https://doi.org/10.1016/S0378-5173(99)00147-7
Bois FY, Tozer TN, Hauck WW, Chen M-L, Patnaik R, Williams RL (1994) Bioequivalence: performance of several measures of extent of absorption. Pharm Res 11:715–722. https://doi.org/10.1023/A:1018932430733
Connarn JN, Flowers S, Kelly M, Luo R, Ward KM, Harrington G, Moncion I, Kamali M, McInnis M, Feng MR et al (2017) Pharmacokinetics and pharmacogenomics of bupropion in three different formulations with different release kinetics in healthy human volunteers. AAPS J 19:1513–1522. https://doi.org/10.1208/s12248-017-0102-8
Hsieh N-H, Reisfeld B, Bois FY, Chiu WA (2018) Applying a global sensitivity analysis workflow to improve the computational efficiencies in physiologically-based pharmacokinetic modeling. Front Pharmacol 9:588. https://doi.org/10.3389/fphar.2018.00588
Saltelli A, Tarantola S, Chan K-S (1999) A quantitative model-independent method for global sensitivity analysis of model output. Technometrics 41:39–56. https://doi.org/10.1080/00401706.1999.10485594
Sarrazin F, Pianosi F, Wagener T (2016) Global sensitivity analysis of environmental models: convergence and validation. Environ Model Softw 79:135–152. https://doi.org/10.1016/j.envsoft.2016.02.005
Zurlinden TJ, Reisfeld B (2017) Characterizing the effects of race/ethnicity on acetaminophen pharmacokinetics using physiologically based pharmacokinetic modeling. Eur J Drug Metab Pharmacokinet 42:143–153. https://doi.org/10.1007/s13318-016-0329-2
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB (2013) Bayesian data analysis. Chapman; Hall/CRC
Bois FY (2013) Bayesian inference. In: Reisfeld B, Mayeno AN (eds) Computational toxicology. Methods in molecular biology (methods and protocols). Humana Press, Totowa, pp 597–636
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. https://doi.org/10.1214/ss/1177011136
Gelman A (2006) Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian Anal 1:515–534. https://doi.org/10.1214/06-BA117A
Chow S-C, Wang H (2001) On sample size calculation in bioequivalence trials. J Pharmacokinet Pharmacodyn 28:155–169. https://doi.org/10.1023/A:1011503032353
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Hsieh N-H, Reisfeld B, Chiu WA (2020) pksensi: An R package to apply global sensitivity analysis in physiologically based kinetic modeling. SoftwareX 12:100609
Bois FY (2009) GNU MCSim: Bayesian statistical inference for SBML-coded systems biology models. Bioinformatics 25:1453–1454. https://doi.org/10.1093/bioinformatics/btp162
RStudio Team (2019) RStudio: integrated development environment for r. RStudio Inc, Boston
Loisios-Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J (2020) Establishing virtual bioequivalence and clinically relevant specifications using in vitro biorelevant dissolution testing and physiologically-based population pharmacokinetic modelling. Case example: naproxen. Eur J Pharm Sci 143:105170. https://doi.org/10.1016/j.ejps.2019.105170
Jereb R, Opara J, Legen I, Petek B, Grabnar-Peklar D (2020) In vitro–in vivo relationship and bioequivalence prediction for modified-release capsules based on a PBPK absorption model. AAPS PharmSciTech 21:18. https://doi.org/10.1208/s12249-019-1566-x
Mitra A (2019) Maximizing the role of physiologically based oral absorption modeling in generic drug development. Clin Pharmacol Ther 105:307–309. https://doi.org/10.1002/cpt.1242
Mitra A, Petek B, Bajc A, Velagapudi R, Legen I (2019) Physiologically based absorption modeling to predict bioequivalence of controlled release and immediate release oral products. Eur J Pharm Biopharm 134:117–125. https://doi.org/10.1016/j.ejpb.2018.11.019
Pepin XJ, Flanagan TR, Holt DJ, Eidelman A, Treacy D, Rowlings CE (2016) Justification of drug product dissolution rate and drug substance particle size specifications based on absorption PBPK modeling for lesinurad immediate release tablets. Mol Pharm 13:3256–3269. https://doi.org/10.1021/acs.molpharmaceut.6b00497
Babiskin AH, Zhang X (2015) Application of physiologically based absorption modeling for amphetamine salts drug products in generic drug evaluation. J Pharm Sci 104:3170–3182. https://doi.org/10.1002/jps.24474
Chung JI, Kelly RC, Wahlstrom J, Wu B, Wu T, Alvarez-Nunez F (2017) Maximizing the impact of physiologically based oral absorption modeling and simulation. J Pharm Sci 106:734–737. https://doi.org/10.1016/j.xphs.2016.11.015
Liu D, Li L, Rostami-Hodjegan A, Bois FY, Jamei M (2020) Considerations and caveats when applying global sensitivity analysis methods to physiologically based pharmacokinetic models. AAPS J 22:1–13. https://doi.org/10.1208/s12248-020-00480-x
McNally K, Cotton R, Loizou GD (2011) A workflow for global sensitivity analysis of PBPK models. Front Pharmacol 2:31. https://doi.org/10.3389/fphar.2011.00031
Melillo N, Aarons L, Magni P, Darwich AS (2019) Variance based global sensitivity analysis of physiologically based pharmacokinetic absorption models for BCS i–IV drugs. J Pharmacokinet Pharmacodyn 46:27–42. https://doi.org/10.1007/s10928-018-9615-8
Ramteke K, Dighe P, Kharat A, Patil S (2014) Mathematical models of drug dissolution: a review. Sch Acad J Pharm 3:388–396. https://doi.org/10.1016/j.ijpharm.2013.04.044
Yang X, Duan J, Fisher J (2016) Application of physiologically based absorption modeling to characterize the pharmacokinetic profiles of oral extended release methylphenidate products in adults. PLoS ONE. https://doi.org/10.1371/journal.pone.0164641
Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, Tucker G (2009) Population-based mechanistic prediction of oral drug absorption. AAPS J 11:225–237. https://doi.org/10.1208/s12248-009-9099-y
Dolton MJ, Chiang P-C, Ma F, Jin JY, Chen Y (2020) A physiologically based pharmacokinetic model of vismodegib: deconvoluting the impact of saturable plasma protein binding, pH-dependent solubility and nonsink permeation. AAPS J 22:1–10. https://doi.org/10.1208/s12248-020-00503-7
Acknowledgements
Funding for this work was made possible, in part, by the U.S. Environmental Protection Agency (STAR RD84003201), U.S Food and Drug Administration (1U01FD005838) and U.S. National Institute of Environmental Health Sciences (P42 ES027704). This article reflects the views of the author and should not be construed to represent FDA’s views or policies. Views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organizations imply endorsement by the United States Government.
Author information
Authors and Affiliations
Contributions
N-HH, FYB, ET, and WAC conceived the overall research concept and design. N-HH performed most analyses, simulations, and preliminary manuscript writing. FYB developed the physiologically based pharmacokinetic absorption model and most computational experiment design. ET, ZN, MY, WS, and MK from the U.S. FDA reviewed the manuscript and provide critical comments during the whole research period. BR is the main funding acquisition and project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Frédéric Y. Bois is currently employed by the CERTARA company but has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hsieh, NH., Bois, F.Y., Tsakalozou, E. et al. A Bayesian population physiologically based pharmacokinetic absorption modeling approach to support generic drug development: application to bupropion hydrochloride oral dosage forms. J Pharmacokinet Pharmacodyn 48, 893–908 (2021). https://doi.org/10.1007/s10928-021-09778-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-021-09778-5