Abstract
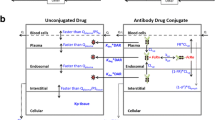
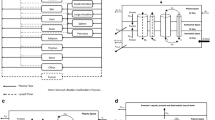
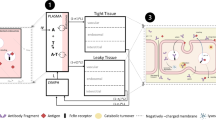
In the past, our lab proposed a two-pore PBPK model for different-size protein therapeutics using de novo derived parameters and the model was validated using plasma PK data of different-size antibody fragments digitized from the literature (Li Z, Shah DK, J Pharmacokinet Pharmacodynam 46(3):305–318, 2009). To further validate the model using tissue distribution data, whole-body biodistribution study of 6 different-size proteins in mice were conducted. Studied molecules covered a wide MW range (13–150 kDa). Plasma PK and tissue distribution profiles is 9 tissues were measured, including heart, lung, liver, spleen, kidney, skin, muscle, small intestine, large intestine. Tumor exposure of different-size proteins were also evaluated. The PBPK model was validated by comparing percentage predictive errors (%PE) between observed and model predicted results for each type of molecule in each tissue. Model validation showed that the two-pore PBPK model was able to predict plasma, tissues and tumor PK of all studied molecules relatively well. This model could serve as a platform for developing a generic PBPK model for protein therapeutics in the future.














Similar content being viewed by others
References
Li Z, Shah DK (2019) Two-pore physiologically based pharmacokinetic model with de novo derived parameters for predicting plasma PK of different size protein therapeutics. J Pharmacokinet Pharmacodynam 46(3):305–318
U.S. Food and Drug Administration (2018) Physiologically based pharmacokinetic analyses—format and content guidance for industry. Center for Drug Evaluation and Research, Silver Spring
Glassman PM, Balthasar JP (2019) Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development. Drug Metab Pharmacokinet 34(1):3–13
Covell DG, Barbet J, Holton OD, Black CD, Parker R, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F (ab′) 2, and Fab′ in mice. Cancer Res 46(8):3969–3978
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54(6):1517–1528
Ferl GZ, Wu AM, DiStefano JJ (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33(11):1640–1652
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodynam 34(5):687–709
Shah DK, Haddish-Berhane N, Betts A (2012) Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodynam 39(6):643–659
Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O (2001) Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int 60(5):1885–1892
Boswell CA, Tesar DB, Mukhyala K, Theil F-P, Fielder PJ, Khawli LA (2010) Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem 21(12):2153–2163
Newkirk M, Novick J, Stevenson M, FOURNIER MJ, Apostolakos P, (1996) Differential clearance of glycoforms of IgG in normal and autoimmune-prone mice. Clin Exp Immunol 106(2):259–264
Shah DK, Betts AM (2013) Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. In: MAbs, 2013, vol 2, Taylor & Francis, pp 297–305
Li Z, Krippendorff B-F, Sharma S, Walz AC, Lavé T, Shah DK (2016) Influence of molecular size on tissue distribution of antibody fragments. In: MAbs, 2016, vol 1, Taylor & Francis, pp 113–119
Li Z, Krippendorff B-F, Shah DK (2017) Influence of Molecular size on the clearance of antibody fragments. Pharm Res 34(10):2131–2141
Rippe B, Haraldsson B (1994) Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74(1):163–219
Rippe B, Haraldsson B (1987) Fluid and protein fluxes across small and large pores in the microvasculature: application of two-pore equations. Acta Physiol Scand 131(3):411–428
Davda JP, Jain M, Batra SK, Gwilt PR, Robinson DH (2008) A physiologically based pharmacokinetic (PBPK) model to characterize and predict the disposition of monoclonal antibody CC49 and its single chain Fv constructs. Int Immunopharmacol 8(3):401–413
Baxter LT, Zhu H, Mackensen DG, Butler WF, Jain RK (1995) Biodistribution of monoclonal antibodies: scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res 55(20):4611–4622
Niederalt C, Kuepfer L, Solodenko J, Eissing T, Siegmund H-U, Block M, Willmann S, Lippert J (2018) A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J Pharmacokinet Pharmacodynam 45(2):235–257
Sepp A, Berges A, Sanderson A, Meno-Tetang G (2015) Development of a physiologically based pharmacokinetic model for a domain antibody in mice using the two-pore theory. J Pharmacokinet Pharmacodynam 42(2):97–109
Sepp A, Meno-Tetang G, Weber A, Sanderson A, Schon O, Berges A (2019) Computer-assembled cross-species/cross-modalities two-pore physiologically based pharmacokinetic model for biologics in mice and rats. J Pharmacokinet Pharmacodynam 46(4):339–359
Li Z, Li Y, Chang H-P, Chang H-Y, Guo L, Shah DK (2019) Effect of size on solid tumor disposition of protein therapeutics. Drug Metab Dispos 47(10):1136–1145
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodynam 39(1):67–86
Schmidt MM, Wittrup KD (2009) A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 8(10):2861–2871
Haraldsson B, Nyström J, Deen WM (2008) Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88(2):451–487
Boswell CA, Mundo EE, Ulufatu S, Bumbaca D, Cahaya HS, Majidy N, Van Hoy M, Schweiger MG, Fielder PJ, Prabhu S (2014) Comparative physiology of mice and rats: radiometric measurement of vascular parameters in rodent tissues. Mol Pharm 11(5):1591–1598
Chang H-P, Kim SJ, Shah DK (2020) Whole-body pharmacokinetics of antibody in mice determined using enzyme-linked immunosorbent assay and derivation of tissue interstitial concentrations. J Pharm Sci
Vegt E, De Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, Gotthardt M, Boerman OC (2010) Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med 51(7):1049–1058
Hong H, Zhang Y, Orbay H, Valdovinos HF, Nayak TR, Bean J, Theuer CP, Barnhart TE, Cai W (2013) Positron emission tomography imaging of tumor angiogenesis with a 61/64Cu-labeled F (ab′) 2 antibody fragment. Mol Pharm 10(2):709–716
Sandström K, Haylock A-K, Spiegelberg D, Qvarnström F, Wester K, Nestor M (2012) A novel CD44v6 targeting antibody fragment with improved tumor-to-blood ratio. Int J Oncol 40(5):1525–1532
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogen Res 2(1):14
Chen N, Wang W, Fauty S, Fang Y, Hamuro L, Hussain A, Prueksaritanont T (2014) The effect of the neonatal Fc receptor on human IgG biodistribution in mice. In: MAbs, 2014, vol 2, Taylor & Francis, pp 502–508
Singh AP, Shah DK (2017) Application of a PK-PD modeling and simulation-based strategy for clinical translation of antibody-drug conjugates: a case study with trastuzumab emtansine (T-DM1). AAPS J 19(4):1054–1070
Acknowledgements
This work was supported by National Institute of General Medical Sciences grant [GM114179]. D.K.S. is also supported by and National Institute of Allergy and Infectious Diseases Grant [AI138195] and National Cancer Institute Grant [R01CA246785].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Z., Li, Y., Chang, H.P. et al. Two-pore physiologically based pharmacokinetic model validation using whole-body biodistribution of trastuzumab and different-size fragments in mice. J Pharmacokinet Pharmacodyn 48, 743–762 (2021). https://doi.org/10.1007/s10928-021-09772-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-021-09772-x