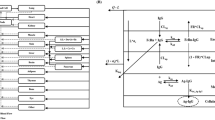
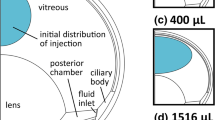
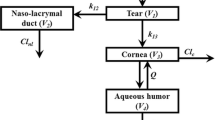
Abstract
Development of protein therapeutics for ocular disorders, particularly age-related macular degeneration (AMD), is a highly competitive and expanding therapeutic area. However, the application of a predictive and translatable ocular PK model to better understand ocular disposition of protein therapeutics, such as a physiologically-based pharmacokinetic (PBPK) model, is missing from the literature. Here, we present an expansion of an antibody platform PBPK model towards rabbit and incorporate a novel anatomical and physiologically relevant ocular component. Parameters describing all tissues, flows, and binding events were obtained from existing literature and fixed a priori. First, translation of the platform PBPK model to rabbit was confirmed by evaluating the model’s ability to predict plasma PK of a systemically administered exogenous antibody. Then, the PBPK model with the new ocular component was validated by estimation of serum and ocular (i.e. aqueous humor, retina, and vitreous humor) PK of two intravitreally administered monoclonal antibodies. We show that the proposed PBPK model is capable of accurately (i.e. within twofold) predicting ocular exposure of antibody-based drugs. The proposed PBPK model can be used for preclinical-to-clinical translation of antibodies developed for ocular disorders, and assessment of ocular toxicity for systemically administered antibody-based therapeutics.






Similar content being viewed by others
References
Gordois A, Pezzullo L, Cutler H (2010) The Global Economic Cost of Visual Impairment. Access Economics Pty Limited for AMD Alliance International
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY (2014) Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2(2):e106–116. https://doi.org/10.1016/S2214-109X(13)70145-1
Global $10.4 Bn Wet Age-Related Macular Degeneration Market to 2024— Rising Prevalence of AMD, Lack of a Specific Treatment, and Increasing Aging Population (2019). GlobeNewswire, 2019/05/15
Hutton-Smith LA, Gaffney EA, Byrne HM, Maini PK, Schwab D, Mazer NA (2016) A Mechanistic model of the intravitreal pharmacokinetics of large molecules and the pharmacodynamic suppression of ocular vascular endothelial growth factor levels by ranibizumab in patients with neovascular age-related macular degeneration. Mol Pharm 13(9):2941–2950. https://doi.org/10.1021/acs.molpharmaceut.5b00849
Hutton-Smith LA, Gaffney EA, Byrne HM, Maini PK, Gadkar K, Mazer NA (2017) Ocular pharmacokinetics of therapeutic antibodies given by intravitreal injection: estimation of retinal permeabilities using a 3-compartment semi-mechanistic model. Mol Pharm 14(8):2690–2696. https://doi.org/10.1021/acs.molpharmaceut.7b00164
Park SJ, Choi Y, Na YM, Hong HK, Park JY, Park KH, Chung JY, Woo SJ (2016) Intraocular pharmacokinetics of intravitreal aflibercept (Eylea) in a rabbit model. Invest Ophthalmol Vis Sci 57(6):2612–2617. https://doi.org/10.1167/iovs.16-19204
Mordenti J, Cuthbertson RA, Ferrara N, Thomsen K, Berleau L, Licko V, Allen PC, Valverde CR, Meng YG, Fei DT, Fourre KM, Ryan AM (1999) Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol 27(5):536–544. https://doi.org/10.1177/019262339902700507
Kheir WJ, Sniegowski MC, El-Sawy T, Li A, Esmaeli B (2014) Ophthalmic complications of targeted cancer therapy and recently recognized ophthalmic complications of traditional chemotherapy. Surv Ophthalmol 59(5):493–502. https://doi.org/10.1016/j.survophthal.2014.02.004
Renouf DJ, Velazquez-Martin JP, Simpson R, Siu LL, Bedard PL (2012) Ocular toxicity of targeted therapies. J Clin Oncol 30(26):3277–3286. https://doi.org/10.1200/JCO.2011.41.5851
Hager T, Seitz B (2013) Ocular side effects of biological agents in oncology: what should the clinician be aware of? Onco Targets Ther 7:69–77. https://doi.org/10.2147/OTT.S54606
Eaton JS, Miller PE, Mannis MJ, Murphy CJ (2015) Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther 31(10):589–604. https://doi.org/10.1089/jop.2015.0064
Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN (1986) Pharmacokinetics of monoclonal immunoglobulin G1, F(ab')2, and Fab' in mice. Cancer Res 46(8):3969–3978
Baxter LT, Zhu H, Mackensen DG, Jain RK (1994) Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res 54(6):1517–1528
Ferl GZ, Wu AM, DiStefano JJ 3rd (2005) A predictive model of therapeutic monoclonal antibody dynamics and regulation by the neonatal Fc receptor (FcRn). Ann Biomed Eng 33(11):1640–1652. https://doi.org/10.1007/s10439-005-7410-3
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34(5):687–709. https://doi.org/10.1007/s10928-007-9065-1
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39(1):67–86. https://doi.org/10.1007/s10928-011-9232-2
del Amo EM, Urtti A (2015) Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res 137:111–124. https://doi.org/10.1016/j.exer.2015.05.003
Short BG (2008) Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol 36(1):49–62. https://doi.org/10.1177/0192623307310955
Pepin-Covatta S, Lutsch C, Grandgeorge M, Lang J, Scherrmann JM (1996) Immunoreactivity and pharmacokinetics of horse anti-scorpion venom F(ab')2-scorpion venom interactions. Toxicol Appl Pharmacol 141(1):272–277. https://doi.org/10.1006/taap.1996.0284
Riviere G, Choumet V, Audebert F, Sabouraud A, Debray M, Scherrmann JM, Bon C (1997) Effect of antivenom on venom pharmacokinetics in experimentally envenomed rabbits: toward an optimization of antivenom therapy. J Pharmacol Exp Ther 281(1):1–8
Ismail M, Abd-Elsalam MA, Al-Ahaidib MS (1998) Pharmacokinetics of 125I-labelled Walterinnesia aegyptia venom and its distribution of the venom and its toxin versus slow absorption and distribution of IGG, F(AB')2 and F(AB) of the antivenin. Toxicon 36(1):93–114. https://doi.org/10.1016/s0041-0101(97)00062-7
Gutierrez JM, Leon G, Lomonte B (2003) Pharmacokinetic-pharmacodynamic relationships of immunoglobulin therapy for envenomation. Clin Pharmacokinet 42(8):721–741. https://doi.org/10.2165/00003088-200342080-00002
Malkevich NV, Basu S, Rudge TL Jr, Clement KH, Chakrabarti AC, Aimes RT, Nabors GS, Skiadopoulos MH, Ionin B (2013) Effect of anthrax immune globulin on response to BioThrax (anthrax vaccine adsorbed) in New Zealand white rabbits. Antimicrob Agents Chemother 57(11):5693–5696. https://doi.org/10.1128/AAC.00460-13
Nagy CF, Mondick J, Serbina N, Casey LS, Carpenter SE, French J, Guttendorf R (2017) Animal-to-human dose translation of obiltoxaximab for treatment of inhalational anthrax under the US FDA animal rule. Clin Transl Sci 10(1):12–19. https://doi.org/10.1111/cts.12433
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ (2007) Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 114(5):855–859. https://doi.org/10.1016/j.ophtha.2007.01.017
Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T (2009) Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci 50(10):4807–4813. https://doi.org/10.1167/iovs.08-3148
Sinapis CI, Routsias JG, Sinapis AI, Sinapis DI, Agrogiannis GD, Pantopoulou A, Theocharis SE, Baltatzis S, Patsouris E, Perrea D (2011) Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin Ophthalmol 5:697–704. https://doi.org/10.2147/OPTH.S19555
Gadkar K, Pastuskovas CV, Le Couter JE, Elliott JM, Zhang J, Lee CV, Sanowar S, Fuh G, Kim HS, Lombana TN, Spiess C, Nakamura M, Hass P, Shatz W, Meng YG, Scheer JM (2015) Design and pharmacokinetic characterization of novel antibody formats for ocular therapeutics. Invest Ophthalmol Vis Sci 56(9):5390–5400. https://doi.org/10.1167/iovs.15-17108
Johnson RL, Gilbert M, Meschia G, Battaglia FC (1985) Cardiac output distribution and uteroplacental blood flow in the pregnant rabbit: a comparative study. Am J Obstet Gynecol 151(5):682–686. https://doi.org/10.1016/0002-9378(85)90165-6
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10(7):1093–1095. https://doi.org/10.1023/a:1018943613122
Gerlowski LE, Jain RK (1983) Physiologically based pharmacokinetic modeling: principles and applications. J Pharm Sci 72(10):1103–1127. https://doi.org/10.1002/jps.2600721003
Sweeney LM, Kirman CR, Gannon SA, Thrall KD, Gargas ML, Kinzell JH (2009) Development of a physiologically based pharmacokinetic (PBPK) model for methyl iodide in rats, rabbits, and humans. Inhal Toxicol 21(6):552–582. https://doi.org/10.1080/08958370802601569
Swartz MA (2001) The physiology of the lymphatic system. Adv Drug Deliv Rev 50(1–2):3–20
Urva SR, Yang VC, Balthasar JP (2010) Physiologically based pharmacokinetic model for T84.66: a monoclonal anti-CEA antibody. J Pharm Sci 99(3):1582–1600. https://doi.org/10.1002/jps.21918
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14. https://doi.org/10.1186/2040-2384-2-14
Szikora B, Hiripi L, Bender B, Kacskovics I, Ilias A (2017) Characterization of the interactions of rabbit neonatal Fc receptor (FcRn) with rabbit and human IgG isotypes. PLoS ONE 12(9):e0185662. https://doi.org/10.1371/journal.pone.0185662
Li Z, Shah DK (2019) Two-pore physiologically based pharmacokinetic model with de novo derived parameters for predicting plasma PK of different size protein therapeutics. J Pharmacokinet Pharmacodyn 46(3):305–318. https://doi.org/10.1007/s10928-019-09639-2
D'Argenio DZ, Schumitzky A, Wang X (2009) ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simiulations Resource, Los Angeles
Macey R, Oster G, Zahnley T (2009) Berkeley Madonna user's guide. University of California, Berkeley
Chakrabarti M, Cheng KT, Spicer KM, Kirsch WM, Fowler SD, Kelln W, Griende S, Nehlsen-Cannarella S, Willerson R, Spicer SS et al (1995) Biodistribution and radioimmunopharmacokinetics of 131I-Ama monoclonal antibody in atherosclerotic rabbits. Nucl Med Biol 22(6):693–697. https://doi.org/10.1016/0969-8051(95)00008-l
Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB (1999) Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther 288(1):371–378
Rippe B, Haraldsson B (1987) Fluid and protein fluxes across small and large pores in the microvasculature. Application of two-pore equations. Acta Physiol Scand 131(3):411–428. https://doi.org/10.1111/j.1748-1716.1987.tb08257.x
Rippe B, Haraldsson B (1994) Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev 74(1):163–219. https://doi.org/10.1152/physrev.1994.74.1.163
Beeram M, Krop IE, Burris HA, Girish SR, Yu W, Lu MW, Holden SN, Modi S (2012) A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2-positive breast cancer. Cancer 118(23):5733–5740. https://doi.org/10.1002/cncr.27622
Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, Tan-Chiu E, Krop IE, Michaelson RA, Girish S, Amler L, Zheng M, Chu YW, Klencke B, O'Shaughnessy JA (2011) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29(4):398–405. https://doi.org/10.1200/JCO.2010.29.5865
Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C, Allen T, Mavroukakis SA, Attia P, Rosenberg SA (2006) Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother 29(4):455–463. https://doi.org/10.1097/01.cji.0000208259.73167.58
Borkar DS, Lacouture ME, Basti S (2013) Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year review. Support Care Cancer 21(4):1167–1174. https://doi.org/10.1007/s00520-012-1645-y
Fraunfelder FT, Fraunfelder FW (2012) Trichomegaly and other external eye side effects associated with epidermal growth factor. Cutan Ocul Toxicol 31(3):195–197. https://doi.org/10.3109/15569527.2011.636118
Struble C, Howard S, Relph J (2014) Comparison of ocular tissue weights (volumes) and tissue collection techniques in commonly used preclinical animal species. Acta Ophthalmol. https://doi.org/10.1111/j.1755-3768.2014.S005.x
del Amo EM, Rimpelä A-K, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M, Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen K-S, Ruponen M, Urtti A (2017) Pharmacokinetic aspects of retinal drug delivery. Progress in Retinal and Eye Research 57:134–185. https://doi.org/10.1016/j.preteyeres.2016.12.001
Nilsson SFE, Alm A (2012) Determination of ocular blood flows with the microsphere method. In: Schmetterer L, Kiel J (eds) Ocular blood flow. Springer, Berlin, pp 25–47. https://doi.org/10.1007/978-3-540-69469-4_2
Fatt I, Hedbys BO (1970) Flow of water in the sclera. Exp Eye Res 10(2):243–249. https://doi.org/10.1016/s0014-4835(70)80035-5
Maurice DM (1987) Flow of water between aqueous and vitreous compartments in the rabbit eye. Am J Physiol 252(1 Pt 2):F104–108. https://doi.org/10.1152/ajprenal.1987.252.1.F104
Prausnitz MR, Noonan JS (1998) Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci 87(12):1479–1488
Wang Q, Chan S, Yang JY, You B, Wang YX, Jonas JB, Wei WB (2016) Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol 168:95–109. https://doi.org/10.1016/j.ajo.2016.05.005
Ames A 3rd, Nesbett FB (1966) Intracellular and extracellular compartments of mammalian central nervous tissue. J Physiol 184(1):215–238. https://doi.org/10.1113/jphysiol.1966.sp007912
Wang JC, Lains I, Providencia J, Armstrong GW, Santos AR, Gil P, Gil J, Talcott KE, Marques JH, Figueira J, Vavvas DG, Kim IK, Miller JW, Husain D, Silva R, Miller JB (2017) Diabetic choroidopathy: choroidal vascular density and volume in diabetic retinopathy with swept-source optical coherence tomography. Am J Ophthalmol 184:75–83. https://doi.org/10.1016/j.ajo.2017.09.030
Mac Gabhann F, Demetriades AM, Deering T, Packer JD, Shah SM, Duh E, Campochiaro PA, Popel AS (2007) Protein transport to choroid and retina following periocular injection: theoretical and experimental study. Ann Biomed Eng 35(4):615–630. https://doi.org/10.1007/s10439-006-9238-x
Acknowledgements
D.K.S is supported by NIH grants GM114179, AI138195, and R01CA246785.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bussing, D., K. Shah, D. Development of a physiologically-based pharmacokinetic model for ocular disposition of monoclonal antibodies in rabbits. J Pharmacokinet Pharmacodyn 47, 597–612 (2020). https://doi.org/10.1007/s10928-020-09713-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-020-09713-0