Abstract
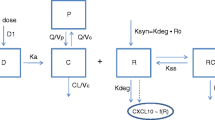
A population pharmacokinetic/pharmacodynamic (popPK/PD) model for BIIB059 (anti-blood dendritic cell antigen 2 [anti-BDCA2]), a humanized immunoglobulin G1 monoclonal antibody currently under development for the treatment of SLE and CLE, is presented. BIIB059 binds BDCA2, a plasmacytoid dendritic cell (pDC)-specific receptor that inhibits the production of IFN-I and other inflammatory mediators when ligated. Phase 1 PK and PD data of healthy adult volunteers (HV, n = 87) and SLE subjects (n = 22) were utilized for the development of the popPK/PD model. The data included single and multiple dosing of intravenous and subcutaneous BIIB059. BDCA2 internalization (PD marker) was measured for all subjects by monitoring reduction of BDCA2 on pDC cell surface and used for development of the popPD model. A two-compartment popPK model with linear plus non-linear elimination was found to best describe BIIB059 PK. BDCA2 levels were best captured using an indirect response model with stimulation of the elimination of BDCA2. Clearance in SLE subjects was 25% higher compared to HV (6.87 vs 5.52 mL/h). Bodyweight was identified as only other covariate on clearance and central volume. The estimates of EC50 and Emax were 0.35 μg/mL and 8.92, respectively. No difference in EC50 and Emax was observed between SLE and HV. The popPK/PD model described the data accurately, as evaluated by pcVPCs and bootstrap. The presented popPK/PD model for BIIB059 provides valuable insight into the dynamics and dose–response relationship of BIIB059 for the treatment of SLE and CLE and was used to guide dose selection for the Phase 2 clinical study (NCT02847598).





Similar content being viewed by others
References
Kyttaris VC (2010) Systemic lupus erythematosus: from genes to organ damage. Methods Mol Biol 662:265–283
Herrmann M, Voll RE, Kalden JR (2000) Etiopathogenesis of systemic lupus erythematosus. Immunol Today 21(9):424–426
Levinsky RJ, Cameron JS, Soothill JF (1977) Serum immune complexes and disease activity in lupus nephritis. Lancet 1(8011):564–567
Yildirim-Toruner C, Diamond B (2011) Current and novel therapeutics in the treatment of systemic lupus erythematosus. J Allergy Clin Immunol 127(2):303–312
Doria A et al (2014) Optimizing outcome in SLE: treating-to-target and definition of treatment goals. Autoimmun Rev 13(7):770–777
Gladman DD et al (2003) Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 30(9):1955–1959
Petri M et al (2014) Burden of corticosteroid use in patients with systemic lupus erythematosus: results from a Delphi panel. Lupus 23(10):1006–1013
Crow MK (2010) Interferon-alpha: a therapeutic target in systemic lupus erythematosus. Rheum Dis Clin North Am 36(1):173–186
Elkon KB, Santer DM (2012) Complement, interferon and lupus. Curr Opin Immunol 24(6):665–670
Khamashta M et al (2016) Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis 75(11):1909–1916
Furie R et al (2017) Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol 69(2):376–386
Siegal FP et al (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284(5421):1835–1837
Blomberg S et al (2001) Presence of cutaneous interferon-alpha producing cells in patients with systemic lupus erythematosus. Lupus 10(7):484–490
Farkas L et al (2001) Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 159(1):237–243
Tomasini D et al (2010) Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner's lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol 37(11):1132–1139
Ghoreishi M, Vera Kellet C, Dutz JP (2012) Type 1 IFN-induced protein MxA and plasmacytoid dendritic cells in lesions of morphea. Exp Dermatol 21(6):417–419
Pellerin A et al (2015) Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms. EMBO Mol Med 7(4):464–476
Dzionek A et al (2001) BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med 194(12):1823–1834
Furie R et al (2019) Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest 129(3):1359–1371
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28(6):507–532
O'Hara DM et al (2011) Recommendations for the validation of flow cytometric testing during drug development: II assays. J Immunol Methods 363(2):120–134
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75(2):85–94
Biliouris K et al (2018) A pre-clinical quantitative model predicts the pharmacokinetics/pharmacodynamics of an anti-BDCA2 monoclonal antibody in humans. J Pharmacokinet Pharmacodyn 45(6):817–827
Jonsson EN, Karlsson MO (1998) Automated covariate model building within NONMEM. Pharm Res 15(9):1463–1468
Bergstrand M et al (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Mould DR, Green B (2010) Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 24(1):23–39
Puchalski T et al (2010) Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res 16(5):1652–1661
Ng CM et al (2006) Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm Res 23(1):95–103
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39(1):67–86
Abuqayyas L, Balthasar JP (2012) Application of PBPK modeling to predict monoclonal antibody disposition in plasma and tissues in mouse models of human colorectal cancer. J Pharmacokinet Pharmacodyn 39(6):683–710
Mager DE (2006) Target-mediated drug disposition and dynamics. Biochem Pharmacol 72(1):1–10
Yan X, Mager DE, Krzyzanski W (2010) Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn 37(1):25–47
McBride JM et al (2012) Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study. Arthritis Rheum 64(11):3666–3676
Chen P et al (2015) Pharmacokinetic and pharmacodynamic relationship of AMG 811, an anti-IFN-gamma IgG1 monoclonal antibody, in patients with systemic lupus erythematosus. Pharm Res 32(2):640–653
Peletier LA, Gabrielsson J (2012) Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification. J Pharmacokinet Pharmacodyn 39(5):429–451
Acknowledgements
This study was supported by Biogen. We acknowledge David Dai for his initial contribution to the concept and the analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
HN, LS, CS, DR, and IAN were employed by Biogen when this work was performed. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views or position of any of their employers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hartmann, S., Biliouris, K., Naik, H. et al. A clinical population pharmacokinetic/pharmacodynamic model for BIIB059, a monoclonal antibody for the treatment of systemic and cutaneous lupus erythematosus. J Pharmacokinet Pharmacodyn 47, 255–266 (2020). https://doi.org/10.1007/s10928-020-09688-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-020-09688-y