Abstract
Amiodarone is a first-line antiarrhythmic for life-threatening ventricular fibrillation or ventricular tachycardia in children, yet little is known about its pharmacokinetics (PK) in this population. We developed a population PK (PopPK) model using samples collected via an opportunistic study design of children receiving amiodarone per standard of care supplemented by amiodarone PK data from the literature. Both study data and literature data were predominantly from infants < 2 years old, so our analysis was restricted to this group. The final combined dataset consisted of 266 plasma drug concentrations in 45 subjects with a median (interquartile range) postnatal age of 40.1 (11.0–120.4) days and weight of 3.9 (3.1–5.1) kg. Since the median sampling time after the first dose was short (study: 95 h; literature: 72 h) relative to the terminal half-life estimated in adult PopPK studies, values of the deep compartment volume and flow were fixed to literature values. A 3-compartment model best described the data and was validated by visual predictive checks and non-parametric bootstrap analysis. The final model included body weight as a covariate on all volumes and on both inter-compartmental and elimination clearances. The empiric Bayesian estimates for clearance (CL), volume of distribution at steady state, and terminal half-life were 0.25 (90% CL 0.14–0.36) L/kg/h, 93 (68–174) L/kg, and 266 (197–477) h, respectively. These studies will provide useful information for future PopPK studies of amiodarone in infants and children that could improve dosage regimens.




Similar content being viewed by others
References
Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, Berg RA, Registry National, of Cardiopulmonary Resuscitation Investigators (2006) First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA 295:50–57
Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA, American Heart Association National Registry of CPR Investigators (2006) Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med 354:2328–2339
Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, Hickey RW, Marino BS, Nadkarni VM, Proctor LT, Qureshi FA, Sartorelli K, Topjian A, van der Jagt EW, Zaritsky AL (2010) Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 122:S876–S908
Silva JN, Erickson CC, Carter CD, Greene EA, Kantoch M, Collins KK, Miyake CY, Carboni MP, Rhee EK, Papez A, Anand V, Bowman TM, Van Hare GF, Participating Members of Pediatric and Congenital Electrophysiology Society (PACES) (2014) Management of pediatric tachyarrhythmias on mechanical support. Circ Arrhythm Electrophysiol 7:658–663
Etheridge SP, Craig JE, Compton SJ (2001) Amiodarone is safe and highly effective therapy for supraventricular tachycardia in infants. Am Heart J 141:105–110
Burri S, Hug MI, Bauersfeld U (2003) Efficacy and safety of intravenous amiodarone for incessant tachycardias in infants. Eur J Pediatr 162:880–884
Elsherbiny ME, El-Kadi AO, Brocks DR (2008) The metabolism of amiodarone by various CYP isoenzymes of human and rat, and the inhibitory influence of ketoconazole. J Pharm Pharm Sci 11:147–159
Pollak PT, Bouillon T, Shafer SL (2000) Population pharmacokinetics of long-term oral amiodarone therapy. Clin Pharmacol Ther 67:642–652
Chow MS (1996) Intravenous amiodarone: pharmacology, pharmacokinetics, and clinical use. Ann Pharmacother 30:637–643
Freedman MD, Somberg JC (1991) Pharmacology and pharmacokinetics of amiodarone. J Clin Pharmacol 31:1061–1069
Araki R, Yukawa E, Nakashima MN, Fukuchi H, Sasaki H, Yano K, Nakashima M (2011) Population pharmacokinetic investigation for optimization of amiodarone therapy in Japanese patients. Ther Drug Monit 33:750–756
Kannan R, Miller S, Singh BN (1985) Tissue uptake and metabolism of amiodarone after chronic administration in rabbits. Drug Metab Dispos 13:646–650
Korth-Bradley JM, Rose GM, de Vane PJ, Peters J, Chiang ST (1996) Population pharmacokinetics of intravenous amiodarone in patients with refractory ventricular tachycardia/fibrillation. J Clin Pharmacol 36:715–719
Plomp TA, van Rossum JM, Robles de Medina EO, van Lier T, Maes RA (1984) Pharmacokinetics and body distribution of amiodarone in man. Arzneimittelforschung 34:513–520
Riva E, Gerna M, Latini R, Giani P, Volpi A, Maggioni A (1982) Pharmacokinetics of amiodarone in man. J Cardiovasc Pharmacol 4:264–269
Vadiei K, Troy S, Korth-Bradley J, Chiang ST, Zimmerman JJ (1997) Population pharmacokinetics of intravenous amiodarone and comparison with two-stage pharmacokinetic analysis. J Clin Pharmacol 37:610–617
Veronese ME, McLean S, Hendriks R (1988) Plasma protein binding of amiodarone in a patient population: measurement by erythrocyte partitioning and a novel glass-binding method. Br J Clin Pharmacol 26:721–731
Wyss PA, Moor MJ, Bickel MH (1990) Single-dose kinetics of tissue distribution, excretion and metabolism of amiodarone in rats. J Pharmacol Exp Ther 254:502–507
Kowey PR, Marinchak RA, Rials SJ, Bharucha D (1997) Pharmacologic and pharmacokinetic profile of class III antiarrhythmic drugs. Am J Cardiol 80:16g–23g
Chang PM, Silka MJ, Moromisato DY, Bar-Cohen Y (2010) Amiodarone versus procainamide for the acute treatment of recurrent supraventricular tachycardia in pediatric patients. Circ Arrhythm Electrophysiol 3:134–140
Dick M, Scott WA (1986) Amiodarone in pediatric patients. Clin Prog Electrophysiol Pacing 4:522–527
Dieks JK, Klehs S, Müller MJ, Paul T, Krause U (2016) Adjunctive ivabradine in combination with amiodarone: a novel therapy for pediatric congenital junctional ectopic tachycardia. Heart Rhythm 13:1297–1302
Dubin AM, Van Hare GF, Collins KK, Bernstein D, Rosenthal DN (2001) Survey of current practices in use of amiodarone and implantable cardioverter defibrillators in pediatric patients with end-stage heart failure. Am J Cardiol 88:809–810
Fishberger SB, Hannan RL, Welch EM, Rossi AF (2009) Amiodarone for pediatric resuscitation: a word of caution. Pediatr Cardiol 30:1006–1008
Haas NA, Camphausen CK (2008) Impact of early and standardized treatment with amiodarone on therapeutic success and outcome in pediatric patients with postoperative tachyarrhythmia. J Thorac Cardiovasc Surg 136:1215–1222
Hasegawa T, Oshima Y, Maruo A, Matsuhisa H, Kadowaki T, Noda R (2013) Landiolol for junctional ectopic tachycardia refractory to amiodarone after pediatric cardiac surgery. Gen Thorac Cardiovasc Surg 61:350–352
Hatib NA, Lee JH, Loh LE, Mok YH, Menon AP, Loh TF, Tan TH (2016) Accidental intra-arterial infusion of amiodarone in a pediatric patient with atrial ectopic tachycardia. Crit Care Med 44:e1013–e1014
Perry JC, Fenrich AL, Hulse JE, Triedman JK, Friedman RA, Lamberti JJ (1996) Pediatric use of intravenous amiodarone: efficacy and safety in critically Ill patients from a multicenter protocol. J Am Coll Cardiol 27:1246–1250
Mazic U, Berden P, Podnar T (2004) Repetitive paroxysms of supraventricular tachyarrhythmias triggered during pediatric cardiac interventions: suppression after short infusion of amiodarone. Pediatr Cardiol 25:684–685
Moffett BS, Valdes SO, Kim JJ (2013) Amiodarone monitoring practices in pediatric hospitals in the United States. Pediatr Cardiol 34:1762–1766
Nalli N, Stewart-Teixeira L, Dipchand AI (2006) Amiodarone-sirolimus/tacrolimus interaction in a pediatric heart transplant patient. Pediatr Transplant 10:736–739
Paul T, Guccione P (1994) New antiarrhythmic drugs in pediatric use: amiodarone. Pediatr Cardiol 15:132–138
Perry JC, Fenrich AL, Hulse JE, Triedman JK, Friedman RA, Lamberti JJ (1996) Pediatric use of intravenous amiodarone: efficacy and safety in critically ill patients from a multicenter protocol. J Am Coll Cardiol 27:1246–1250
Ramusovic S, Läer S, Meibohm B, Lagler FB, Paul T (2013) Pharmacokinetics of intravenous amiodarone in children. Arch Dis Child 98:989–993
Saharan S, Balaji S (2015) Cardiovascular collapse during amiodarone infusion in a hemodynamically compromised child with refractory supraventricular tachycardia. Ann Pediatr Cardiol 8:50–52
Saul JP, Scott WA, Brown S, Marantz P, Acevedo V, Etheridge SP, Perry JC, Triedman JK, Burriss SW, Cargo P, Graepel J, Koskelo EK, Wang R, Intravenous Amiodarone Pediatric Investigators (2005) Intravenous amiodarone for incessant tachyarrhythmias in children: a randomized, double-blind, antiarrhythmic drug trial. Circulation 112:3470–3477
Tsuda E, Matsuo M, Sakaguchi H, Hayashi T, Hosoda K, Miyazaki A (2010) Combined amiodarone and low-dose carvedilol treatment for severe heart failure in childhood. Pediatr Int 52:e39–e42
Valdes SO, Donoghue AJ, Hoyme DB, Hammond R, Berg MD, Berg RA, Samson RA, American Heart Association Get With The Guidelines-Resuscitation Investigators (2014) Outcomes associated with amiodarone and lidocaine in the treatment of in-hospital pediatric cardiac arrest with pulseless ventricular tachycardia or ventricular fibrillation. Resuscitation 85:381–386
Gonzalez D, Melloni C, Yogev R, Poindexter BB, Mendley SR, Delmore P, Sullivan JE, Autmizguine J, Lewandowski A, Harper B, Watt KM, Lewis KC, Capparelli EV, Benjamin DK Jr, Cohen-Wolkowiez M, Pharmaceuticals Best, for Children Act—Pediatric Trials Network Administrative Core Committee (2014) Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther 96:429–437
Holford N, Heo Y, Anderson B (2013) A pharmacokinetic standard for babies and adults. J Pharm Sci 102:2941–2952
Weibel ER (2002) Physiology: the pitfalls of power laws. Nature 417:131–132
Anderson BJ, Holford NH (2008) Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332
Dennis J, Schnabel RB (1996) Numerical methods for unconstrained optimization and nonlinear equations. Class Appl Math 10(1137/1):9781611971200
Upton RN (2004) Calculating the hybrid (macro) rate constants of a three-compartment mamillary pharmacokinetic model from known micro-rate constants. J Pharmacol Toxicol Methods 49:65–68
Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT (2011) Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics 3:53–72
Batchelor HK, Marriott JF (2015) Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 79:395–404
Holt DW, Tucker GT, Jackson PR, Storey GC (1983) Amiodarone pharmacokinetics. Am Heart J 106:840–847
Hines RN (2013) Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm 452:3–7
Acknowledgements
This work was funded under National Institute of Child Health and Human Development contract HHSN275201000003I for the Pediatric Trials Network (Principal Investigator Daniel K. Benjamin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The assay measuring amiodarone concentrations was performed at OpAns Laboratory (Durham, NC).
The PTN publications committee
Gary Furda, Duke Clinical Research Institute, Durham, NC; Daniel K. Benjamin, Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, UC San Diego, San Diego, CA; Gregory L. Kearns, Arkansas Children’s Hospital Research Institute, Little Rock, AR; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Christoph P. Hornik, Duke Clinical Research Institute, Durham, NC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development
David Siegel and Perdita Taylor-Zapata
The EMMES Corporation (Data Coordinating Center)
Ravinder Anand and Gina Simone
Pediatric trials network amiodarone study team, principal investigators (PIs), and study coordinators (SCs)
Duke Clinical Research Institute: Tammy Day (clinical research associate). Medical University of South Carolina Children’s Hospital: Hibah Al Nasiri (SC), Ann Frampton (SC), Patricia Infinger (SC). Ann and Robert H. Lurie Children’s Hospital of Chicago: Laura Fearn (SC). University of Louisville-KCPCRU and Norton Children’s Hospital: Tressa Bratton (SC), Jacqueline Perry (SC), Jennifer Comings (SC). Oregon Health and Science University: Kira Clark (SC). University of Maryland: Donna Cannonier (SC). University of North Carolina at Chapel Hill: Janice Bernhardt (SC). The Children’s Hospital Colorado: Peter Mourani (Site PI), Susan Gunn (SC). University of Virginia Children’s Hospital: Michelle Adu-Darko (Site PI), Robin Kelly (SC). Rainbow Babies and Children’s Hospital: Stuart Goldstein (Site PI), Tara Terrell (SC). Duke University Medical Center: Kevin Watt (Site PI), Samantha Wrenn (SC), Christie Milleson (SC).
Disclosures
S.H.D. receives support from the National Institutes of Health (NIH)/National Institute of General Medicine Sciences (T32GM086330). C.P.H receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). M.C.W. receives support for research from the NIH (1R01-HD076676-01A1), the National Institute of Allergy and Infectious Diseases (HHSN272201500006I and HHSN272201300017I), the National Institute of Child Health and Human Development (HHSN275201000003I), the Biomedical Advanced Research and Development Authority (HHSO100201300009C), and industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). The other authors have no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10928_2018_9576_MOESM2_ESM.pdf
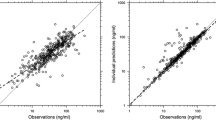
Supplementary material Fig. 1 Standard goodness-of-fit plots of the final model depicting only the PTN dataset. (The same data are represented by Fig. 1 but are presented here because the PTN data points are largely obscured in that figure by the larger number of data points from the Ramusovic dataset.) Observed versus population (A) and individual (B) predictions for the amiodarone model. The line of identity is indicated by a solid line. Conditional weighted residuals (CWRES) versus time after dose (C) and population predictions (D) for the base amiodarone PK model (PDF 73 kb)
10928_2018_9576_MOESM3_ESM.pdf
Supplementary material Fig. 2 Observed serum amiodarone concentrations in each patient versus time (hours). In subjects with more than one concentration, the model-predicted concentration–time course is illustrated with a solid line for the duration of the drug administration (PDF 655 kb)
Rights and permissions
About this article
Cite this article
Dallefeld, S.H., Atz, A.M., Yogev, R. et al. A pharmacokinetic model for amiodarone in infants developed from an opportunistic sampling trial and published literature data. J Pharmacokinet Pharmacodyn 45, 419–430 (2018). https://doi.org/10.1007/s10928-018-9576-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-018-9576-y