Abstract
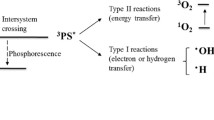
The combination of photodynamic therapy (PDT) with anti-tumor agents is a complimentary strategy to treat local cancers. We developed a unique photosensitizer (PS)-conjugated paclitaxel (PTX) prodrug in which a PS is excited by near-infrared wavelength light to site-specifically release PTX while generating singlet oxygen (SO) to effectively kill cancer cells with both PTX and SO. The aim of the present study was to identify the determinants influencing the combined efficacy of this light-activatable prodrug, especially the bystander killing effects from released PTX. Using PS-conjugated PTX as a model system, we developed a quantitative mathematical model describing the intracellular trafficking. Dynamics of the prodrug and the model predictions were verified with experimental data using human cancer cells in vitro. The sensitivity analysis suggested that parameters related to extracellular concentration of released PTX, prodrug uptake, target engagement, and target abundance are critical in determining the combined killing efficacy of the prodrug. We found that released PTX cytotoxicity was most sensitive to the retention time of the drug in extracellular space. Modulating drug internalization and conjugating the agents targeted to abundant receptors may provide a new strategy for maximizing the killing capacity of the far-red light-activatable prodrug system. These results provide guidance for the design of the PDT combination study in vivo and have implications for other stimuli-responsive drug delivery systems.






Similar content being viewed by others
References
Au JL, Yeung BZ, Wientjes MG, Lu Z, Wientjes MG (2016) Delivery of cancer therapeutics to extracellular and intracellular targets: determinants, barriers, challenges and opportunities. Adv Drug Deliv Rev 97:280–301. doi:10.1016/j.addr.2015.12.002
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul 41:189–207
Siegel RA (2014) Stimuli sensitive polymers and self regulated drug delivery systems: a very partial review. J Control Release 190:337–351. doi:10.1016/j.jconrel.2014.06.035
Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M (2014) A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochem Biophys Acta 1846 1:75–87. doi:10.1016/j.bbcan.2014.04.005
Sievers EL, Senter PD (2013) Antibody-drug conjugates in cancer therapy. Annu Rev Med 64:15–29. doi:10.1146/annurev-med-050311-201823
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12(11):991–1003. doi:10.1038/nmat3776
Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T (2010) Development and applications of photo-triggered theranostic agents. Adv Drug Deliv Rev 62(11):1094–1124. doi:10.1016/j.addr.2010.09.002
Liu Y, Gunda V, Zhu X, Xu X, Wu J, Askhatova D, Farokhzad OC, Parangi S, Shi J (2016) Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer. Proc Natl Acad Sci USA 113(28):7750–7755. doi:10.1073/pnas.1605841113
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A (2011) Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103(2):317–324. doi:10.1007/s11060-010-0389-0
Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC (2013) Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Can Res 73(8):2412–2417. doi:10.1158/0008-5472.CAN-12-4561
Yao JH, Feng JX, Chen J (2016) External-stimuli responsive systems for cancer theranostic. Asian J Pharm Sci 11(5):585–595. doi:10.1016/j.ajps.2016.06.001
Alvarez-Lorenzo C, Bromberg L, Concheiro A (2009) Light-sensitive intelligent drug delivery systems. Photochem Photobiol 85(4):848–860. doi:10.1111/j.1751-1097.2008.00530.x
Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A (2010) Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol Membr Biol 27(7):364–381. doi:10.3109/09687688.2010.507788
Milligan PA, Brown MJ, Marchant B, Martin SW, van der Graaf PH, Benson N, Nucci G, Nichols DJ, Boyd RA, Mandema JW, Krishnaswami S, Zwillich S, Gruben D, Anziano RJ, Stock TC, Lalonde RL (2013) Model-based drug development: a rational approach to efficiently accelerate drug development. Clin Pharmacol Ther 93(6):502–514. doi:10.1038/clpt.2013.54
van der Graaf PH, Benson N (2011) Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm Res 28(7):1460–1464. doi:10.1007/s11095-011-0467-9
Sharan S, Woo S (2014) Quantitative insight in utilizing circulating angiogenic factors as biomarkers for antiangiogenic therapy: systems pharmacology approach. CPT 3:e139. doi:10.1038/psp.2014.36
Thapa P, Li M, Bio M, Rajaputra P, Nkepang G, Sun Y, Woo S, You Y (2016) Far-red light-activatable prodrug of paclitaxel for the combined effects of photodynamic therapy and site-specific paclitaxel chemotherapy. J Med Chem 59(7):3204–3214. doi:10.1021/acs.jmedchem.5b01971
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer Inst 90(12):889–905. doi:10.1093/jnci/90.12.889
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther 1(4):279–293. doi:10.1016/S1572-1000(05)00007-4
Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 6(7):535–545. doi:10.1038/nrc1894
Rajaputra P, Bio M, Nkepang G, Thapa P, Woo S, You Y (2016) Anticancer drug released from near IR-activated prodrug overcomes spatiotemporal limits of singlet oxygen. Bioorg Med Chem 24(7):1540–1549. doi:10.1016/j.bmc.2016.02.025
Jusko WJ (1989) Pharmacokinetics of capacity-limited systems. J Clin Pharmacol 29(6):488–493. doi:10.1002/j.1552-4604.1989.tb03369.x
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34(5):711–726. doi:10.1007/s10928-007-9066-0
Soriano J, Stockert JC, Villanueva A, Canete M (2010) Cell uptake of Zn(II)-phthalocyanine-containing liposomes by clathrin-mediated endocytosis. Histochem Cell Biol 133(4):449–454. doi:10.1007/s00418-010-0679-9
Kuh HJ, Jang SH, Wientjes MG, Au JL (2000) Computational model of intracellular pharmacokinetics of paclitaxel. J Pharmacol Exp Ther 293(3):761–770
Jang SH, Wientjes MG, Au JL (2001) Kinetics of P-glycoprotein-mediated efflux of paclitaxel. J Pharmacol Exp Ther 298(3):1236–1242
Sutherland RL, Hall RE, Taylor IW (1983) Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Can Res 43(9):3998–4006
Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12(1):1–10. doi:10.1208/s12248-009-9155-7
Soetaert K, Petzoldt T (2010) Inverse modelling, sensitivity and monte carlo analysis in R using package FME. J Stat Softw 33(3):1–28
Correia RF, Andrade SM, Viseu MI (2012) Aggregation and disaggregation of anionic aluminum phthalocyanines in cationic pre-micelle and micelle media: a fluorescence study. J Photochem Photobiol A 235:21–28. doi:10.1016/j.jphotochem.2012.03.002
Rejman J, Oberle V, Zuhorn IS, Hoekstra D (2004) Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 377(Pt 1):159–169. doi:10.1042/BJ20031253
Frohlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7:5577–5591. doi:10.2147/IJN.S36111
Bio M, Rajaputra P, Nkepang G, You Y (2014) Far-red light activatable, multifunctional prodrug for fluorescence optical imaging and combinational treatment. J Med Chem 57(8):3401–3409. doi:10.1021/jm5000722
Gomez-Millan J, Katz IS, Farias Vde A, Linares-Fernandez JL, Lopez-Penalver J, Ortiz-Ferron G, Ruiz-Ruiz C, Oliver FJ, Ruiz de Almodovar JM (2012) The importance of bystander effects in radiation therapy in melanoma skin-cancer cells and umbilical-cord stromal stem cells. Radiother Oncol 102(3):450–458. doi:10.1016/j.radonc.2011.11.002
Dahle J, Bagdonas S, Kaalhus O, Olsen G, Steen HB, Moan J (2000) The bystander effect in photodynamic inactivation of cells. Bba-Gen Subj 1475(3):273–280. doi:10.1016/S0304-4165(00)00077-5
Eatock MM, Schatzlein A, Kaye SB (2000) Tumour vasculature as a target for anticancer therapy. Cancer Treat Rev 26(3):191–204. doi:10.1053/ctrv.1999.0158
Chaplin DJ, Pettit GR, Parkins CS, Hill SA (1996) Antivascular approaches to solid tumour therapy: evaluation of tubulin binding agents. Br J Cancer Suppl 27:S86–S88
Tozer GM, Prise VE, Wilson J, Cemazar M, Shan S, Dewhirst MW, Barber PR, Vojnovic B, Chaplin DJ (2001) Mechanisms associated with tumor vascular shut-down induced by combretastatin A-4 phosphate: intravital microscopy and measurement of vascular permeability. Can Res 61(17):6413–6422
Folkman J (1997) Angiogenesis and angiogenesis inhibition: an overview. Exs 79:1–8
Siemann DW (2011) The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev 37(1):63–74. doi:10.1016/j.ctrv.2010.05.001
Denekamp J (1982) Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br J Cancer 45(1):136–139
Martinelli M, Bonezzi K, Riccardi E, Kuhn E, Frapolli R, Zucchetti M, Ryan AJ, Taraboletti G, Giavazzi R (2007) Sequence dependent antitumour efficacy of the vascular disrupting agent ZD6126 in combination with paclitaxel. Br J Cancer 97(7):888–894. doi:10.1038/sj.bjc.6603969
Ensminger WD, Gyves JW (1984) Regional cancer chemotherapy. Cancer Treat Rep 68(1):101–115
Siemann DW, Chaplin DJ, Walicke PA (2009) A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P). Expert Opin Investig Drugs 18(2):189–197. doi:10.1517/13543780802691068
Wang W, Moriyama LT, Bagnato VS (2013) Photodynamic therapy induced vascular damage: an overview of experimental PDT. Laser Phys Lett 10(2):023001. doi:10.1088/1612-2011/10/2/023001
Kim YW, Bae SM, Battogtokh G, Bang HJ, Ahn WS (2012) Synergistic anti-tumor effects of combination of photodynamic therapy and arsenic compound in cervical cancer cells: in vivo and in vitro studies. PLoS ONE 7(6):e38583. doi:10.1371/journal.pone.0038583
Huang HC, Mallidi S, Liu J, Chiang CT, Mai Z, Goldschmidt R, Ebrahim-Zadeh N, Rizvi I, Hasan T (2016) Photodynamic therapy synergizes with irinotecan to overcome compensatory mechanisms and improve treatment outcomes in pancreatic cancer. Can Res 76(5):1066–1077. doi:10.1158/0008-5472.CAN-15-0391
Gazi L, Bobirnac I, Danzeisen M, Schupbach E, Langenegger D, Sommer B, Hoyer D, Tricklebank M, Schoeffter P (1999) Receptor density as a factor governing the efficacy of the dopamine D4 receptor ligands, L-745,870 and U-101958 at human recombinant D4.4 receptors expressed in CHO cells. Brotosh J Pharmacol 128(3):613–620. doi:10.1038/sj.bjp.0702849
Copeland RA, Pompliano DL, Meek TD (2006) Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov 5(9):730–739. doi:10.1038/nrd2082
Tachibana R, Harashima H, Shono M, Azumano M, Niwa M, Futaki S, Kiwada H (1998) Intracellular regulation of macromolecules using pH-sensitive liposomes and nuclear localization signal: qualitative and quantitative evaluation of intracellular trafficking. Biochem Biophys Res Commun 251(2):538–544. doi:10.1006/bbrc.1998.9460
Kim H, Chung K, Lee S, Kim DH, Lee H (2016) Near-infrared light-responsive nanomaterials for cancer theranostics. Wiley Interdiscip Rev Nanomed Nanobiotechnol 8(1):23–45. doi:10.1002/wnan.1347
Shubayev VI, Pisanic TR 2nd, Jin S (2009) Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev 61(6):467–477. doi:10.1016/j.addr.2009.03.007
Deelman LE, Decleves AE, Rychak JJ, Sharma K (2010) Targeted renal therapies through microbubbles and ultrasound. Adv Drug Deliv Rev 62(14):1369–1377. doi:10.1016/j.addr.2010.10.002
Schmaljohann D (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58(15):1655–1670. doi:10.1016/j.addr.2006.09.020
Li R, Feng F, Wang Y, Yang X, Yang X, Yang VC (2014) Folic acid-conjugated pH/temperature/redox multi-stimuli responsive polymer microspheres for delivery of anti-cancer drug. J Colloid Interface Sci 429:34–44. doi:10.1016/j.jcis.2014.05.008
Su Y, Hu Y, Du Y, Huang X, He J, You J, Yuan H, Hu F (2015) Redox-responsive polymer-drug conjugates based on doxorubicin and chitosan oligosaccharide-g-stearic acid for cancer therapy. Mol Pharm 12(4):1193–1202. doi:10.1021/mp500710x
Gupta P, Vermani K, Garg S (2002) Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov Today 7(10):569–579. doi:10.1016/S1359-6446(02)02255-9
Nkepang G, Bio M, Rajaputra P, Awuah SG, You Y (2014) Folate receptor-mediated enhanced and specific delivery of far-red light-activatable prodrugs of combretastatin A-4 to FR-positive tumor. Bioconjug Chem 25(12):2175–2188. doi:10.1021/bc500376j
Breskey JD, Lacey SE, Vesper BJ, Paradise WA, Radosevich JA, Colvard MD (2013) Photodynamic therapy: occupational hazards and preventative recommendations for clinical administration by healthcare providers. Photomed Laser Surg 31(8):398–407. doi:10.1089/pho.2013.3496
Zhu TC, Liu B, Penjweini R (2015) Study of tissue oxygen supply rate in a macroscopic photodynamic therapy singlet oxygen model. J Biomed Optics 20(3):038001. doi:10.1117/1.JBO.20.3.038001
Zhu TC, Finlay JC, Zhou X, Li J (2007) Macroscopic modeling of the singlet oxygen production during PDT. In Proceedings of SPIE–the international society for optical engineering 6427:642708. doi:10.1117/12.701387
Cilliers C, Guo H, Liao JS, Christodolu N, Thurber GM (2016) Multiscale modeling of antibody-drug conjugates: connecting tissue and cellular distribution to whole animal pharmacokinetics and potential implications for efficacy. AAPS J 18(5):1117–1130. doi:10.1208/s12248-016-9940-z
Acknowledgements
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM113940, Department of Defense Breast Cancer Research Program under Award Number W81XWH-09-1-0071, and the Presbyterian Health Foundation.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, M., Thapa, P., Rajaputra, P. et al. Quantitative modeling of the dynamics and intracellular trafficking of far-red light-activatable prodrugs: implications in stimuli-responsive drug delivery system. J Pharmacokinet Pharmacodyn 44, 521–536 (2017). https://doi.org/10.1007/s10928-017-9543-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-017-9543-z