Abstract
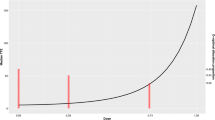
The objective of this paper was to find and investigate the performance of the D optimal designs for three Poisson dose–response models. Phase II dose ranging studies are pivotal in the drug development program, being used to select dose(s) for phase III. Count data is encountered in a number of clinical areas. The Poisson distribution provides an intuitive platform for modelling such data, especially when combined with random effects which allow subjects to differ in their response rates. This work investigated three Poisson dose–response models of increasing complexity. A simple Emax model was used to describe the drug effect, and D optimal designs under a range of different parameter values (scenarios) were found. The relative performances between scenarios were assessed using: the precision of all parameters, the precision of the drug effect parameters, and the percent coefficient of variation (%CV) of the ED50 parameter. The results showed that the D optimal designs were similar across models and scenarios, with the D optimal designs consisting of placebo, the maximum dose, and a dose just below the ED50. However the relative performance of the optimal designs was very different. For example, with 1,000 subjects, the %CV of the ED50 parameter ranged from 1.4 to 91 %. Performance typically improved with higher baseline counts, smaller random effects, and larger Emax. This work introduces a framework for determining and evaluating the performance of D optimal designs for phase II dose ranging studies with count data as the primary endpoint.





Similar content being viewed by others
References
Sheiner LB (1997) Learning versus confirming in clinical drug development. Clin Pharmacol Ther 61(3):275–291
Snoeck E, Stockis A (2007) Dose-response population analysis of levetiracetam add-on treatment in refractory epileptic patients with partial onset seizures. Epilepsy Res 73(3):284–291
Gupta SK, Sathyan G, Lindemulder EA, Ho PL, Sheiner LB, Aarons L (1999) Quantitative characterization of therapeutic index: application of mixed-effects modeling to evaluate oxybutynin dose-efficacy and dose-side effect relationships. Clin Pharmacol Ther 65(6):672–684
Poisson SD (1837) Probabilité jugements en matière criminelle et en matière civile précédées règles générales calcul probabilitiés (Paris). Bachelier, Paris, p 206
Atkinson AC, Donev AN (1992) Optimum experimental designs. (Oxford statistical science series; 8). Oxford University Press, Oxford
Chaloner K, Verdinelli I (1995) Bayesian experimental design: a review. Stat Sci 10(3):273–304
Mentre F, Mallet A, Baccar D (1997) Optimal design in random-effects regression models. Biometrika 84(2):429–442
Ogungbenro K, Aarons L (2008) Optimisation of sampling windows design for population pharmacokinetic experiments. J Pharmacokinet Pharmacodyn 35(4):465–482
Chenel M, Bouzom F, Cazade F, Ogungbenro K, Aarons L, Mentré F (2008) Drug-drug interaction predictions with PBPK models and optimal multiresponse sampling time designs: application to midazolam and a phase I compound. Part 2: clinical trial results. J Pharmacokinet Pharmacodyn 35(6):661–681
Khinkis LA, Krzyzanski W, Jusko WJ, Greco WR (2009) D-optimal designs for parameter estimation for indirect pharmacodynamic response models. J Pharmacokinet Pharmacodyn 36(6):523–539
Silber HE, Nyberg J, Hooker AC, Karlsson MO (2009) Optimization of the intravenous glucose tolerance test in T2DM patients using optimal experimental design. J Pharmacokinet Pharmacodyn 36(3):281–295
Plan EL, Maloney A, Trocóniz IF, Karlsson MO (2009) Performance in population models for count data, part I: maximum likelihood approximations. J Pharmacokinet Pharmacodyn 36(4):353–366
Ogungbenro K, Aarons L (2010) Sample size/power calculations for population pharmacodynamic experiments involving repeated-count measurements. J Biopharm Stat 20(5):1026–1042
Ogungbenro K, Aarons L (2011) Population Fisher information matrix and optimal design of discrete data responses in population pharmacodynamic experiments. J Pharmacokinet Pharmacodyn 38(4):449–469
Nyberg J, Karlsson MO, Hooker AC (2009) Population optimal experimental design for discrete type data. PAGE (Population Approach Group Europe) 18, St. Petersburgh, Russia Abstr 1468. www.page-meeting.org/?abstract=1468
Fedorov VV (1972) Theory of optimal experiments. Academic Press, New York
SAS Institute Inc., Cary, NC, USA
Maloney A, Schaddelee M, Freijer J, Krauwinkel W, van Gelderen M, Jacqmin P, Simonsson US (2010) An example of optimal phase II design for exposure response modelling. J Pharmacokinet Pharmacodyn 37(5):475–491
Maloney A, Karlsson MO, Simonsson US (2007) Optimal adaptive design in clinical drug development: a simulation example. J Clin Pharmacol 47(10):1231–1243
Acknowledgments
The authors would like to thank two reviewers for a number of important suggestions which significantly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The individual components on the expected FIM matrix for model 1 were determined analytically. The expected FIM matrix for model 1 is:
where LL is the log-likelihood. For the Poisson model, the log likelihood is:
For x counts, and for model 1:
Thus
By noting that E(x) = \( \lambda \), the above expressions can be simplified and used to determine the expected FIM. Thus we obtain:
So finally the expected FIM can be written as:
Rights and permissions
About this article
Cite this article
Maloney, A., Simonsson, U.S.H. & Schaddelee, M. D optimal designs for three Poisson dose–response models. J Pharmacokinet Pharmacodyn 40, 201–211 (2013). https://doi.org/10.1007/s10928-013-9300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-013-9300-x