Abstract
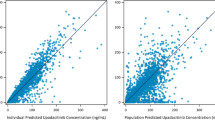
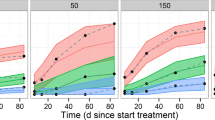
The objective of this study was to provide support for a body weight-tiered dosing regimen by characterizing abatacept pharmacokinetics (PK) and the relationship between exposure and the ACR20 (American College of Rheumatology criteria for 20% improvement) response in Japanese patients with rheumatoid arthritis (RA). A population PK model was developed using NONMEM with 2,535 samples from 344 Japanese RA patients in two clinical trials. The exposure–response relationship was characterized using a Generalized Estimating Equation (GEE) logistic regression model, with time-varying actual trough concentrations and ACR20 responder rates over 6 months in a randomized, placebo-controlled phase 2 trial for stable methotrexate. Abatacept exposure was well characterized using a linear, two-compartment model, in which body weight and the empirically calculated glomerular filtration rate were significant covariates for clearance. The ACR20 response model was developed by examining the quasi-likelihood information criterion, and the cumulative logit in the final model was specified by the log-transformed trough concentration. The predicted ACR20 responder rate was consistent with the actual values in the clinical trial and this model revealed trough concentrations higher than the recommended body weight-tiered dose are unlikely to result in substantial increases in clinical efficacy. Considering that ACR20 is a longitudinal binary variable and the response to RA treatment is delayed, the GEE model was useful for predicting the probability of an ACR20 response. In conclusion, the same dosing regimen as non-Japanese patients is recommended because a body weight-tiered dosing regimen achieves similar exposures across the wide range of body weight.








Similar content being viewed by others
References
Genovese MC, Becker J-C, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M (2005) Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353:1114–1123
Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, Weiner RS, Birkhofer MJ, Warner GL, Berry KK, Linsley PS, Krueger JG, Ochs HD, Kelley SL, Kang S (1999) CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest 103:1243–1252
Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S, Russell A, Dougados M, Emery P, Nuamah IF, Williams GR, Becker J-C, Hagerty DT, Moreland LW (2003) Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 349:1907–1915
Goldring SR (2002) Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin Rheumatol 14:406–410
Hoffman RW (2001) T cells in the pathogenesis of systemic lupus erythematosus. Front Biosci 6:D1369–D1378
Goronzy JJ, Weyand CM (2004) T-cell regulation in rheumatoid arthritis. Curr Opin Rheumatol 16:212–217
Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14:233–258
Silver PB, Hathcock KS, Chan CC, Wiggert B, Caspi RR (2000) Blockade of costimulation through B7/CD28 inhibits experimental autoimmune uveoretinitis, but does not induce long-term tolerance. J Immunol 165:5041–5047
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916
Maxwell LJ, Singh JA (2010) Abatacept for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol 37:234–245
Ma Y, Lin B-R, Lin B, Hou S, Qian W-Z, Li J, Tan M, Ma J, Li B-H, Wang H, Wen A-D, Guo Y-J (2009) Pharmacokinetics of CTLA4Ig fusion protein in healthy volunteers and patients with rheumatoid arthritis. Acta Pharmacol Sin 30:364–371
Davis PM, Nadler SG, Rouleau KA, Suchard SJ (2005) Abatacept (CTLA4-Ig) modulates human T-cell proliferation and cytokine production but does not affect lipopolysaccharide-induced tumor necrosis factor alpha production by monocytes. Arthritis Res Ther 7(suppl 1):21
Roy A, Mould DR, Wang XF, Tay L, Raymond R, Pfister M (2007) Modeling and simulation of abatacept exposure and interleukin-6 response in support of recommended doses for rheumatoid arthritis. J Clin Pharmacol 47:1408–1420
ICON (2007) NONMEM7™ user’s guide. http://www.globomaxnm.com/nonmem.htm. Accessed Mar 2011
Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA (1984) Regression modeling strategies for improved prognostic prediction. Stat Med 3:143–152
Bradburn MJ, Clark TG, Love SB, Altman DG (2003) Survival analysis part III: multivariate data analysis—choosing a model and assessing its adequacy and fit. Br J Cancer 89:605–611
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F (2007) Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53:766–772
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T, Moriyama T, Ando Y, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S (2007) Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 11:41–50
Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV (2003) Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol 43:610–623
Liang KY, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Lee H, Kimko HC, Rogge M, Wang D, Nestorov I, Peck CC (2003) Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin Pharmacol Ther 73:348–365
Pan W (2001) Akaike’s information criterion in generalized estimating equations. Biometrics 57:120–125
Yasuoka Y, Goto A, Seriu T (2011) Pharmacological properties and clinical efficacy of abatacept (Orencia®) for the treatment of rheumatoid arthritis. Nippon Yakurigaku Zasshi 137:87–94 Japanese
Ternant D, Aubourg A, Magdelaine-Beuzelin C, Degenne D, Watier H, Picon L, Paintaud G (2008) Infliximab pharmacokinetics in inflammatory bowel disease patients. Ther Drug Monit 30:523–529
Weisman MH, Moreland LW, Furst DE, Weinblatt ME, Keystone EC, Paulus HE, Teoh LS, Velagapudi RB, Noertersheuser PA, Granneman GR, Fischkoff SA, Chartash EK (2003) Efficacy, pharmacokinetic, and safety assessment of adalimumab, a fully human anti-tumor necrosis factor-alpha monoclonal antibody, in adults with rheumatoid arthritis receiving concomitant methotrexate: a pilot study. Clin Ther 25:1700–1721
Bristol-Myers K.K. (2010) Orencia (abatacept) [prescribing information]. http://www.orencia.jp. Accessed Mar 2011
Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS (2001) Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 41:1206–1214
Tang L, Persky AM, Hochhaus G, Meibohm B (2004) Pharmacokinetic aspects of biotechnology products. J Pharm Sci 93:2184–2204
Lobo ED, Hansen RJ, Balthasar JP (2004) Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 93:2645–2668
Sun YN, Lu JF, Joshi A, Compton P, Kwon P, Bruno RA (2005) Population pharmacokinetics of efalizumab (humanized monoclonal anti-CD11a antibody) following long-term subcutaneous weekly dosing in psoriasis subjects. J Clin Pharmacol 45:468–476
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28:171–192
Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, Furst D, Goldsmith C, Kieszak S, Lightfoot R, Paulus H, Tugwell P, Weinblatt M, Widmark R, Williams HJ, Wolfe F (1993) The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 36:729–740
Hempel G, Karlsson MO, de Alwis DP, Toublanc N, McNay J, Schaefer HG (1998) Population pharmacokinetic-pharmacodynamic modeling of moxonidine using 24-hour ambulatory blood pressure measurements. Clin Pharmacol Ther 64:622–635
Trocóniz IF, de Alwis DP, Tillmann C, Callies S, Mitchell M, Schaefer HG (2000) Comparison of manual versus ambulatory blood pressure measurements with pharmacokinetic-pharmacodynamic modeling of antihypertensive compounds: application to moxonidine. Clin Pharmacol Ther 68:18–27
Mandema JW, Stanski DR (1996) Population pharmacodynamic model for ketorolac analgesia. Clin Pharmacol Ther 60:619–635
Holford NH, Peace KE (1992) Methodologic aspects of a population pharmacodynamic model for cognitive effects in Alzheimer patients treated with tacrine. Proc Natl Acad Sci USA 89:11466–11470
Gomeni R, Teneggi V, Iavarone L, Squassante L, Bye A (2001) Population pharmacokinetic-pharmacodynamic model of craving in an enforced smoking cessation population: indirect response and probabilistic modeling. Pharm Res 18:537–543
Acknowledgments
We have no conflict of interest regarding our article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hasegawa, M., Imai, Y., Hiraoka, M. et al. Model-based determination of abatacept exposure in support of the recommended dose for Japanese rheumatoid arthritis patients. J Pharmacokinet Pharmacodyn 38, 803–832 (2011). https://doi.org/10.1007/s10928-011-9221-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-011-9221-5