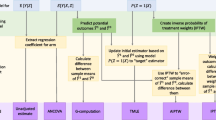
Abstract
Literature data are often reported as multiple (longitudinal) mean outcomes observed in several groups of patients within a study. Observations within a study are correlated because the patients come from a common population, and the mean observations over time within a treatment arm are correlated because they are based on the same set of patients. As a result, model-based meta-analysis may require more than two levels of random effects to correctly characterize this correlation structure. Using simulation, we explored and evaluated ways to implement multi-level random effects in NONMEM. Simulation models that were linear and non-linear in the random effects were investigated. We compared estimation models that included study and/or treatment arm-level random effects, with and without residual correlation. With all estimation strategies, the fixed random effects parameters were accurately estimated. With regard to correctly characterizing the variability, models that accounted for correlation within a study and treatment arm over time were the best in some situations, while models that accounted for study-level correlation only were better in others. Models that included only treatment arm-level random effects were not superior in any scenario.






Similar content being viewed by others
References
Glass GV (1976) Primary, secondary and meta-analysis of research. Educ Res 5:3–8
Piedbois P, Buyse M (2004) Meta-analyses based on abstracted data: a step in the right direction, but only a first step. J Clin Oncol 22:3839–3841
Whitehead A (2002) Meta-analysis of controlled clinical trials. Wiley, New York
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, New York
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Houwelingen HCv, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21:589–624
Nam I, Mengersen K, Garthwaite P (2003) Multivariate meta-analysis. Stat Med 22:2309–2333
Ishak KJ, Platt RW, Joseph L, Hanley JA, Caro JJ (2007) Meta-analysis of longitudinal studies. Clin Trials 4:525–539
Peters JL, Mengersen KL (2008) Meta-analysis of repeated measures study designs. J Eval Clin Pract 14:941–950
Jones AP, Riley RD, Williamson PR, Whitehead A (2009) Meta-analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clin Trials 6:16–27
Sutton AJ, Kendrick D, Coupland CAC (2008) Meta-analysis of individual and aggregate-level data. Stat Med 27:651–669
Suttonand AJ, Higgins JPT (2008) Recent developments in meta-analysis. Stat Med 27:625–650
Cook DJ, Sackett DL, Spitzer WO (1995) Methodologic guidelines for systematic reviews of randomized controlled trials in health care from the Potsdam consultation on meta-analysis. J Clin Epidemiol 48:167–171
Mandema JW, Cox E, Alderman J (2005) Therapeutic benefit of eletriptan compared to sumatriptan for the acute relief of migraine pain–results of a model-based meta-analysis that accounts for encapsulation. Cephalalgia 25:715–725
Mandema JW, Hermann D, Wang W, Sheiner T, Milad M, Bakker-Arkema R, Hartman D (2005) Model-based development of gemcabene a new lipid-altering agent. AAPS J 7:E513–E522
Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, Corrigan BW, Lockwood PA, Marshall SA, Benincosa LJ, Tensfeldt TG, Parivar K, Amantea M, Glue P, Koide H, Miller R (2007) Model-based drug development. Clin Pharmacol Ther 82:21–32
Ito K, Ahadieh S, Corrigan B, French J, Fullerton T, Tensfeldt T, Alzheimer's Disease Working Group (2010) A disease progression meta-analysis model in Alzheimer's disease. Alzheimers Dement 6:39–53
Prins MGNH, Haughie S, Johnson P, Martin SW (2009) Use of model based meta-analysis combining patient-level with summary-level data using multilevel random effects to provide a quantitative assessment of the clinical efficacy (IPSS) profile and competitive positioning of a PDE5 inhibitor (UK369,003) for the treatment of benign prostatic hyperplasia (BPH). p 18, vol Abstr 1625, St. Petersburg, Russia
Venkatakrishnan K, Ezzet F, Ravva P, Tensfeldt TG, Chow V, Chew RD, Barbee T, Dvorak RV, Taylor AE, Obourn JD, Benincosa LJ (2008) Longitudinal dose-response analysis of the effects of sibutramine, orlistat and rimonabant on body weight progression in pooled obesity clinical trials: illustration of knowledge management in model-based drug development. Clin Pharmacol Ther 83(1):PII-45
Beal SL, Sheiner LB, Boeckmann AJ (eds) (1989–2006) NONMEM users guides, ICON Development Solutions, Ellicott City, MD
Laporte-Simitsidis S, Girard P, Mismetti P, Chabaud S, Decousus H, Boissel JP (2000) Inter-study variability in population pharmacokinetic meta-analysis: when and how to estimate it? J Pharm Sci 89:155–167
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735–750
Karlsson MO, Beal SL, Sheiner LB (1995) Three new residual error models for population PK/PD analyses. J Pharmacokinet Biopharm 23:651–672
Silber HE, Kjellsson MC, Karlsson MO (2009) The impact of misspecification of residual error or correlation structure on the type I error rate for covariate inclusion. J Pharmacokinet Pharmacodyn 36:81–99
Agresti A (1990) Categorical data analysis. Wiley, New York
Karlsson MO, Holford NH (2008) A tutorial on visual predictive checks. p 17 Abstr 1434. www.page-meeting.org/?abstract=1434
Wakefield J (2008) Ecologic studies revisited. Annu Rev Public Health 29:75–90
Ishak KJ, Robert WP, Lawrence J, James AH (2008) Impact of approximating or ignoring within-study covariances in multivariate meta-analyses. Stat Med 27:670–686
Ian RW, Julian PTH, Angela MW (2008) Allowing for uncertainty due to missing data in meta-analysis-Part 1: two-stage methods. Stat Med 27:711–727
Ian RW, Nicky JW, Angela MW, Ades AE, Julian PTH (2008) Allowing for uncertainty due to missing data in meta-analysis-Part 2: hierarchical models. Stat Med 27:728–745
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Example NONMEM code for A1

Appendix 2: Example NONMEM code for A2

Rights and permissions
About this article
Cite this article
Ahn, J.E., French, J.L. Longitudinal aggregate data model-based meta-analysis with NONMEM: approaches to handling within treatment arm correlation. J Pharmacokinet Pharmacodyn 37, 179–201 (2010). https://doi.org/10.1007/s10928-010-9152-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-010-9152-6