Abstract
The scientific community has become interested in cellulose extraction from agro-industrial wastes because they contain large quantities of cellulose and are being researched globally due to their application. Cellulose extracted from these wastes offers varying characteristics and structures depending on the specific source. In this study, microcrystalline cellulose (MCC) was extracted from different parts of the durian husk through acid hydrolysis with various concentrations of oxalic acid and cellulose-to-acid ratios. The durian husk underwent a pretreatment method involving bleaching and alkaline treatment. The characteristics of MCC, such as functional group, morphology, and crystallinity, were studied. The findings indicated that lignin was successfully removed through pretreatment. However, traces of hemicellulose were still detected in a few samples. The extracted MCCs demonstrated a diameter range from 6.237 to 25.38 μm and crystallinity within the range of 71.43–78.30%. Polycaprolactone (PCL)-based biocomposites with different MCC weights (0.2, 0.5, and 1.0 wt%) were fabricated, and the performance was evaluated through tensile testing and biodegradability tests. The addition of 1.0 wt% MCC enhanced the tensile strength by 22%. SEM analysis revealed the cross-section fracture surface of the biocomposite, indicating the contribution of the MCC during pull-out. Meanwhile, the biodegradability of the biocomposite increased with the addition of MCC, indicating that the MCC-based biocomposite was fully biodegradable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The adverse impacts of population growth on our natural environment are increasingly evident and have imposed significant stress on the Earth’s ecosystems. The production of waste is one of the most pressing issues. Around the world, one-third of the food produced is wasted, producing 1.3 billion tons of biowaste [1]. Most of the biomass waste is generated from the agricultural-food industry. The growing population and increasing global consumption of plastic materials have also led to significant plastic waste issues. Eight to nine million tons of plastic, mainly from single-use items, end up in the ocean each year [2]. As a result, one million seabirds and 100 million marine species die due to the plastic in the ocean [3]. At this rate, more plastic might be in our ocean than fish by 2050 [4]. Correspondingly, the development of novel sustainable polymers created using natural origin is of growing interest due to environmental issues and consumer requirements for higher quality and longer shelf life of food [5].
Cellulose is the primary component of all plant materials and is consistently regenerated by photosynthesis. About 1/2 to 1/3 of all plant tissue comprises cellulose, which confers stability and strength to the plant cell walls [6]. Cellulose present in plant material can be divided into amorphous and crystalline regions. Cellulose is predominantly composed of the crystalline structure, while the amorphous region makes up a relatively minor proportion [7]. Applying suitable treatments, such as enzymes, chemicals, or mechanical processes, to lignocellulosic biomass can effectively isolate cellulose with a crystalline structure, producing microcrystalline cellulose (MCC) [8]. MCC can subsequently be utilized as a source to produce cellulose nanocrystals (CNC).
MCC is a finely textured, odorless, and white substance, presenting itself as a crystalline powder with excellent mechanical strength, low density, biocompatibility, non-toxicity, reusability, and biodegradability [9]. These characteristics render MCC highly desirable as the reinforcing filler in polymer blends over alternative fibers [10]. It is also recognized as the most robust and rigid natural reinforcing filler, primarily attributed to its large surface area compared to other typical cellulose fibers [11]. The readily available MCC in markets is predominantly derived from virgin sources, such as purified cotton or expensive hardwood. As a result, current research efforts have shifted toward discovering various underutilized lignocellulosic materials biomass waste as inexpensive, diverse, and sustainable sources of MCC. This research work aims to produce MCC by converting waste by-products into value-added products [12].
Durian, known as the “king of fruits”, scientifically referred to as Durio zibethius, belongs to the family Bombacaceae and genus Durio. It is a tropical fruit with a skin full of sharp thorns. Durian rind, also known as durian husk, makes up over 50% of the durian’s quality, and the inner white part of the durian husk is referred to as the white sac [13]. Durian is one of the most popular tropical fruits in Southeast Asia, including Malaysia, Philippines, Indonesia, and Thailand [14]. Collectively, these countries produce over 1,600,000 tons of durian each year.
Moreover, the global durian market, valued at USD 25.01 billion in 2023, is expected to grow substantially with a projected compound annual growth rate of 7% [15]. The rapid urbanization in emerging economies causing the rise of disposable incomes has fueled an expansion of the middle-class demographic. As a result, there is increased demand for premium food products, such as durian pastries and desserts. However, the rise in durian food processing, including the production of durian pulp and paste, has led to an accumulation of non-consumable durian husk waste, presenting potential environmental challenges that must be addressed [16]. One way to tackle this problem is by transforming durian husk waste into valuable resources. Durian husk is a lignocellulosic biomass comprising three primary components of approximately 30.7% hemicellulose, 15.6% lignin, and ranging from 57 to 64% cellulose [14]. The high cellulose content found in durian husk presents an opportunity to extract valuable MCC. The common method used in most research to extract MCC is acid hydrolysis using sulfuric acid. However, sulfuric acid is a strong acid and has several significant disadvantages, such as being hazardous, corrosive, and having environmental compatibility issues [17]. Consequently, exploring alternative milder acids, such as oxalic acid, for MCC extraction is advisable. While there is limited research using oxalic acid for MCC extraction from durian husk, determining the appropriate acid concentration, temperature, and cellulose-to-acid ratio is a task to be undertaken in this study.
In response to the global plastic waste problem, researchers and industries are increasingly exploring the potential of biopolymers or biodegradable polymers, which are derived from living things such as plants and microorganisms. These polymers can be found naturally in human bodies or chemically synthesized from vegetable oils, fats, proteins, or amino acids. This distinction between synthetic and natural polymers arises from their production methods and varied sources [18]. In recent years, synthetic biopolymers have garnered attention because of their notable benefits in flexibility and stability that suit a wide range of applications. Polycaprolactone (PCL), polyglycolic acid (PGA), polydioxanone (PDS), polylactic acid (PLA), and polypropylene fumarate (PPF) are some examples of synthetic biodegradable polymers [19]. Despite the eco-friendly nature of biopolymers, their inherent characteristics typically result in lower mechanical strength and stiffness compared to conventional plastics. However, previous research works have demonstrated the extraction of microcrystalline cellulose from biomass, showcasing its potential as a promising reinforcing agent for biopolymers [20; 21]. Despite the advancements in MCC extraction, previous studies have primarily relied on strong acids, raising concerns about safety and environmental impact. Additionally, many of these studies focused solely on a single set of operating parameters on acid hydrolysis, leaving a knowledge gap in understanding the effects of various conditions on MCC extraction and its performance as a reinforcing agent.
Hence, the present study aims to investigate the feasibility of durian rind to produce microcrystalline cellulose (MCC) as a natural reinforcing agent for biocomposite applications. MCC was extracted from durian rind through a series of chemical pre-treatments followed by oxalic acid hydrolysis at different acid concentrations and cellulose-to-acid ratios. The effectiveness of MCC for biocomposite applications was tested by incorporating it with PCL, evaluating its mechanical properties, biodegradability, and thermal stability.
Methodology
Materials
The durian husk was obtained from a local fruit farm in Kuching, Sarawak. Sodium hydroxide (Quality Reagent Chemical), aqueous glacial acetic acid (HmbG Chemicals), sodium chloride (Quality Reagent Chemicals), sodium hypochlorite (Bendosen), oxalic acid (Merck), PCL (poly(3-caprolactone), and tert-butanol (Merck) were used in this study.
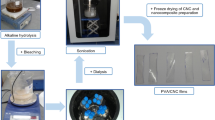
Experimental Flowchart
The MCC extraction process in this study (Fig. 1) starts with an alkaline treatment to remove hemicellulose from the durian rind, followed by a bleaching treatment to remove the lignin. To study the effect of a bleaching agent on the removal of lignin, two routes were applied: the combination of sodium chloride (NaCl) and acetic acid (CH3COOH), and sodium hypochlorite (NaClO). The bleached cellulose then underwent acid hydrolysis with varying conditions to study the yield and purity of the extracted MCC. The cellulose-to-acid ratio is one of the crucial factors for MCC extraction; hence, ratios of 1:10 and 1:50 were used in this study. Additionally, different oxalic acid concentrations [30, 50, and 65% (w/v)] were also examined to evaluate the impact of acid hydrolysis on MCC properties. The extracted MCC was characterized through various analyses. Subsequently, the MCC was mixed with polycaprolactone (PCL) to fabricate biocomposites and further evaluated their mechanical properties, biodegradability, and thermal stability.
Durian Rind Moisture Content
Different parts of durian rind, i.e., durian husk (DH) and white sac (WS), were cut into smaller pieces and washed thoroughly under running water to remove contaminants. The initial mass of DH and WS was weighed and recorded as D1 and dried for 24 h at 100 ºC in a Memmert oven [22]. The final mass of the dried DH and WS were recorded and designated as D2. Equation (1) was used to calculate the moisture content and Fig. 2 shows the DH and WS pieces before and after drying.
Preparation of Raw Materials
To extract the cellulose, DH and WS were ground using a Fritsch rotor mill. The grinding process for the rotor mill involves using an 8-rib rotor and a 1.0 mm Trapez sieve ring made of special steel to ensure the particle size is 1.0 mm. The ground DH and WS powder (Fig. 3) were kept in a desiccator until further use to prevent exposure to moisture in the air [23].
Alkaline Treatment
Kim et al. [23] reported that the ideal conditions of alkaline pretreatment to remove non-cellulosic materials from biomass are using 0.5–10% (w/v) of sodium hydroxide (NaOH), under a temperature of 60–180 ℃, and 0.5–10% of solid loading. In this study, 10 g of the DH and WS was treated with 100 mL of 2% (w/v) NaOH solution at a temperature between 90 and 100 ℃ for 3 h with continued stirring on the IKA hot plate stirrer. The mixture was then cooled to room temperature (25 ℃) and filtered through distilled water. The pore size of the filter paper used was 125 mm in diameter. After filtering the residue, the filtrate was repeatedly rinsed with distilled water until the pH reached neutral to eliminate the alkali. The resultant residue was dried in the oven for 24 h at 60 ºC. The obtained alkaline-treated DH and WS were then ground using a blender and kept in a desiccator until further use.
Bleaching Treatment
The bleaching process was conducted by mixing the alkaline-treated cellulose with the bleaching agent. Two bleaching agents were used to study the effect of the bleaching agent on further removal of lignin from alkaline-treated cellulose. The first bleaching agent was the combination of 0.50% (v/v) aqueous glacial acetic acid (CH3COOH) and 1.50% (w/v) sodium chloride (NaCl), while the second bleaching agent was 5% (v/v) of sodium hypochlorite (NaClO).
The bleaching process was carried out at 70 ºC for 2 h with constant stirring. The bleaching solution was then cooled to room temperature, and the residue was filtered and washed multiple times with distilled water to obtain a neutral pH residue. The bleached cellulose was dried at 60 °C in an oven for 24 h, followed by grinding in a rotor mill. Figure 4 shows the extracted cellulose using different types of bleaching agents. According to Rahman et al. [22], the yield of the extracted cellulose from DH and WS can be calculated using Eq. (2):
Where \(\:{\text{W}}_{\text{C}\text{e}\text{l}\text{l}\text{u}\text{l}\text{o}\text{s}\text{e}}\) is the weight of extracted cellulose and \(\:{\text{W}}_{\text{D}\text{H}/\text{W}\text{S}\:}\) is the weight of DH or WS.
Microcrystalline Cellulose (MCC) Extraction
To extract crystalline cellulose, oxalic acid was used in this study. Oxalic acid is a weak organic acid that requires a higher temperature (above 80 ℃) for efficient production of crystalline cellulose [24]. Raising the hydrolysis temperature promotes the defibrillation of the feed fiber by reducing the degree of polymerization of cellulose fiber which is beneficial for producing crystalline cellulose. On the other hand, if the reaction time is less than 4 h, the hydrolysis process might not adequately break down the cellulose fiber to extract crystalline cellulose efficiently [25]. Hence, extending the reaction time could boost extraction yield. Nevertheless, it is important to note that prolonging the reaction time may also lead to excessive hydrolysis, ultimately leading to a reduced extraction yield [25]. A study by Fitriani et al. [26] examined the duration of acid hydrolysis on crystalline cellulose properties and found that 3 h is efficient for producing cellulose nanocrystals. The crystalline cellulose underwent 3 h of acid hydrolysis exhibit the highest crystallinity and smallest particle size, and produced a stable aqueous suspension of crystalline cellulose. Therefore, the following hydrolysis settings are established.
Solution with oxalic acid concentrations of 30, 50 and 65% (w/v) were weighed and mixed with distilled water in a 250 mL beaker, respectively. The mixture was then heated on the hot plate with continuously stirring until the oxalic acid was fully dissolved in the distilled water. This is because dehydrated oxalic acid cannot dissolve in distilled water at room temperature. Two different cellulose-to-oxalic acid ratios, 1:50 and 1:10, were used to study the effect of the raw material-to-acid ratio on the extraction yield. Table 1 shows the list of samples. The bleached cellulose and oxalic acid mixture was poured into a test tube and placed inside the water bath, then heated to a temperature of 90 ºC for 3 h. The hydrolysis process was stopped by adding a 10-fold excess of distilled water to the reaction mixture [27]. The resulting mixture was instantly placed in the centrifuge tubes and centrifuged for 10 min at 6000 rpm to separate the MCC and the distilled water [28].
Following centrifugation, the liquid supernatant in the centrifuge tubes was drained, and the remaining MCC suspension was transferred to a dialysis tube. The dialysis tube, filled with the MCC suspension, was immersed in a beaker containing distilled water. The distilled water in the beaker was replaced daily until its pH became neutral. To minimize the suspension agglomeration after acid hydrolysis, the MCC suspension was subjected to 10 min of sonication at 25 ºC using an ultrasonic probe [29].
Subsequently, tert-butanol (t-BuOH) was added as a surfactant to the colloidal suspension before drying the MCC suspension in the oven. The co-solvent was prepared by combining t-BuOH and suspension in a ratio of 1:9, as proposed by Hanif et al. [30]. The mixture was stirred until a homogenous dispersion was achieved. After that, the mixture was poured into a beaker and dried in an oven at 100 ºC for 24 h. Figure 5 illustrates the extracted MCC.
The yield percentage of extracted MCC can be calculated as given by Eq. (3):
where \(\:{\text{W}}_{\text{M}\text{C}\text{C}}\) is the weight of MCC and \(\:{\text{W}}_{\text{c}\text{e}\text{l}\text{l}\text{u}\text{l}\text{o}\text{s}\text{e}\:}\) is the weight of extracted cellulose.
Fourier Transform Infrared (FTIR) Analysis
The functional group changes in the chemical structure of lignin, cellulose, and hemicellulose of the extracted MCC were characterized using a Perkin Elmer Spectrum 3 FTIR spectrometer. The oven-dried MCC samples underwent 16 scans at a resolution of 4 cm−1, over the range of 450 cm−1 to 4000 cm−1 [31].
X-Ray Diffraction (XRD) Analysis
The crystallinity index of the extracted MCC was determined using a PANalytical (X’pert Pro, Netherlands) X-ray diffractometer with Cu Kα radiation (λ = 1.54060Å). The MCC was scanned at a step increment of 0.02° within 2θ range of 7° to 60° with X-ray tube voltage and current of 45 kV and 35 mA, respectively. Equation (4), which uses the Segal approach, was used to estimate the relative crystallinity index of MCC, Crl (%).
where \(\:I\:\) represents the peak intensity corresponding to crystalline cellulose, while \(\:{I}_{am}\) is the peak intensity of the amorphous region.
Ultraviolet-Visible (UV-vis) Analysis
To determine the turbidity of the suspensions, which correlates with the size of MCC, UV-vis spectroscopy was performed using a Perkin Elmer Lambda 35 at room temperature ranging from 200 to 800 nm in transmittance mode [32]. The extracted MCC was mixed with distilled water, then transferred into a cuvette and filled to ¾ with the solution.
Morphological Analysis
Field emission scanning electron microscope (FESEM) (Quanta 400, FEI, Switzerland) was employed to examine the surface morphology of the MCC. The FESEM analysis was conducted using a 2–10 kV acceleration voltage and a 6–10 mm working distance. To prevent charging and ensure high-quality images, the samples were sputter-coated with platinum before analysis [31]. In addition, the MCC particle size distribution was determined using a Malvern Zetasizer Nano ZS.
Fabrication of Biocomposite
To fabricate the PCL/MCC biocomposite specimens, the PCL granules were first melted at 70 ºC in a water bath. MCC powder was gradually added to the molten PCL (Fig. 6a) with continuous stirring to ensure even distribution. The resulting mixture was poured into an aluminum mold and left to solidify at room temperature for 5 to 10 min. Table 2 shows the list of biocomposites PCL samples (Fig. 6b) with varying MCC concentrations (0.2, 0.5, and 1.0 wt%). The “untreated” sample incorporated into the PCL refers to the raw materials, specifically the ground durian rind before any treatment has been applied.
Mechanical Properties Characterization
The mechanical properties of the PCL/MCC biocomposite were evaluated using a Shimadzu universal testing machine (UTM) in accordance with the ASTM D638 [33] standard procedure with some modifications. The PCL/MCC biocomposite samples were mounted in the UTM, and the tester’s grips were positioned at a specified grip separation of 102 mm. The PCL/MCC biocomposite samples were then subjected to tension until failure at a constant speed of 0.5 mm/min [34]. The fractured surface of the biocomposite was examined under a FEI, Quanta 400 FESEM. The samples were coated with platinum to ensure proper electrical conductivity, as reported previously [35].
Biodegradability Test
To examine the biodegradability of the biocomposites, samples were cut to 3 cm × 2 cm, with an average weight of 2 g (Fig. 7a). The initial weight of each sample was measured and recorded. The samples were then buried in the soil at a depth of 2 cm, and the pH was measured to be 6.67 [36]. The samples were left to degrade under aerobic conditions 7, 14, and 28 days. The moisture of the soil was maintained with distilled water (Fig. 7b) [37]. At every specified interval duration, the samples were retrieved from the soil, cleaned with distilled water, dried, and weighed. The biodegradability of the samples was evaluated using Eq. (5).
where \(\:{W}_{1}\:\) and \(\:{W}_{2}\)are the weight of sample before and after biodegradability test.
Thermogravimetric Gravimetric Analysis (TGA)
The thermal stability of the biocomposites was determined using a STA 8000 Simultaneous Thermal Analyzer (Perkin Elmer, Waltham, MA, USA). STA measures the change in weight of a sample as a function of temperature and the data will be used to calculate the maximum mass decomposition corresponding to temperature. The dried composite sample weighing between 7 and 17 mg was placed in a sample pan and heated in an environment of nitrogen gas to ensure the change in weight was due to thermal degradation. The heating rate was 20 °C/min, and the temperature range was 50 to 600 °C [38].
Result and Discussion
Durian Husk Moisture Content
The moisture content was measured on the durian husk (DH) and the white sac (WS). Table 3 presents the total weight loss for both DH and WS samples, revealing an average moisture content of 85.19 ± 0.19% for WS, marginally higher than that of the DH, which was recorded as 83.65 ± 0.54%. The results are quantitatively similar to those reported by Pusparizkita et al. [39], who obtained an average moisture content from DH dried in an oven at 100 ℃, with the highest moisture content of 84.15% for the WS and 82.98% for the outer skin. In this study, WS also has a higher moisture content and lower dry matter content than DH [40]. Conversely, DH has a lower moisture content because it contains more dry matter, such as protein, starch, and cell wall material obtained from photosynthesis and movement in the phloem [41].
Effect of Bleaching Agent on Cellulose Yield
The cellulose extraction is initiated by utilizing the durian husk (DH) of the durian rind with a particle size smaller than 1 mm. The strong cohesion bond between lignin, hemicellulose, and pectin, resulting from the β-1,4 glycoside bond and amorphous structure, was disrupted by a 2% (w/v) NaOH solution for 3 h during the alkaline treatment [42]. The product of the alkaline treatment, including cellulose compounds, forms a brown-colored precipitate attributed to the presence of remaining lignin.
Following the alkaline treatment, the bleaching process was carried out, making the alkaline-treated sample brighter. According to Putri and Kurniyati [43], the bleaching process aims to eliminate the remaining lignin from the cellulose. Color-absorbing molecules, including chromophores, undergo oxidation during bleaching, making them water-soluble and polar. Consequently, the extracted cellulose achieves a whiter and brighter color through the bleaching process.
The results of durian husk powder extraction, as depicted Table 4, demonstrate that the yield of extracted cellulose from DH using sodium chloride (NaCl) and glacial acetic acid was 30.36%. However, using sodium hypochlorite (NaClO) as the bleaching agent resulted in a lower cellulose yield, specifically, 23.82%. These results align with the research conducted by Xing et al. [44], who used the same raw materials but different alkaline and bleaching solutions, resulting in a yield of 32.23%, and the research conducted by Penjumras et al. [45], which utilized durian husk and a different treatment method to obtain a cellulose yield of 33.12 ± 0.108%. It is apparent that NaClO may be more potent in eliminating non-cellulosic compounds from cellulose.
Additionally, using NaClO as a bleaching agent increases the brightness of the cellulose (Fig. 4b), turning the sample white. However, this effect does not occur when using NaCl and glacial acetic acid. The primary reason is that NaClO is a potent bleaching and oxidizing agent [46]. During the bleaching treatment with NaClO, the lignin network is partly degraded into soluble compounds and partly separated from cellulose by breaking the lignin-carbohydrate bonds [47]. It efficiently degrades color-causing substances [48], such as lignin, demonstrating excellent non-lignocellulosic removal efficiency in bleaching treatment. Therefore, more non-cellulosic substances in the raw material led to an enhancement in carboxyl and carbonyl content, as well as an improved whiteness index [49]. On the other hand, sodium chloride is a salt, not an oxidizing agent, and requires the addition of acetic acid to fully penetrate the durian husk [50]. During bleaching, acetic acid is added to sodium chloride to alter the pH to 3 to 4, creating a bleaching environment needed for lignin removal. Since acetic acid is weak, and the amount added to NaCl is minimal, which explains why NaClO is more effective than the NaCl/acetic acid mixture.
In summary, the effectiveness of NaClO as a bleaching agent was compared to mixture NaCl/acetic acid mixture in terms of cellulose yield and purity. The results showed that the NaCl/acetic acid mixture produced a higher cellulose yield of 30.36%, outperforming NaClO, which yielded 23.82%. However, it is crucial to consider additional factors, such as cellulose purity and experimental conditions, when determining the better bleaching agent. It is evident that NaClO is effective in producing high purity cellulose, as indicated by the bleached durian husk turning white due to the elimination of color-causing substances. Although NaClO is a powerful and effective bleaching agent, the specific conditions under which it was used in this study might have led to a lower cellulose yield than the NaCl/acetic acid mixture due to its aggressive action. However, the bleaching effect was better with NaClO than the NaCl/acetic acid mixture.
Effect of Acid Concentration on MCC Yield
One of the crucial factors in the extraction of MCC during acid hydrolysis is acid concentration. The MCC yield significantly decreased when the oxalic acid concentration increased from 30% (w/v) to 65% (w/v), as evident in Table 5. The highest MCC yield of 47.35% was obtained at 30% (w/v), while 65% (w/v) of oxalic acid resulted in a lower MCC extraction yield of 34.39%. These findings confirm that a high oxalic acid concentration is unfavorable for MCC extraction because it leads to the complete degradation of cellulose, producing sugar molecules [51]. According to Tang et al. [52], a higher acid concentration can cause a severe breakdown of cellulose into sugars. Conversely, a lower acid concentration can result in poorly aggregated and scattered fibers. Different raw materials require relatively narrow and specific acid concentration ranges to achieve optimum results. Additional parameters, such as cellulose-to-acid ratio and extraction from various parts of the durian husk, were also considered as they may affect the MCC yield. Further research could be done to optimize the MCC yield by reducing the acid concentration to less than 30% (w/v), as lower acid concentrations have resulted in higher MCC, as shown in Table 5.
Effect of Cellulose-to-Acid Ratio on MCC Yield
Two cellulose-to-acid ratios (1:10 and 1:50) were studied, showing MCC yields ranging from 34.39 to 72.59% for both DH and WS samples (Table 5). When the cellulose-to-acid ratio decreased from 1:50 to 1:10, the MCC yield for DH increased from 47.35 to 72.59%. This result is in line with a study where MCC extracted from sweet sorghum using hydrochloric acid with a cellulose-to-acid ratio ranging from 1:10 to 1:30. Notably, the study reported that a lower cellulose-to-acid ratio appeared to favor a higher MCC yield [53]. This implies that a higher cellulose-to-acid ratio facilitates the hydrolysis reaction more easily. However, the movement of more H+ ions towards the β-1,4-glycosidic link weakens the influence of the acid ion on the breakdown of glycosidic bonds. A similar finding was reported by Doan and Chiang [54], that the yield decreased dramatically from 52 to 31% when the cellulose-to-acid ratio increased from 1:30 to 1:50 (mL/g). This occurs because a higher cellulose-to-acid ratio could enhance the degree of cellulose hydrolysis.
MCC Yield from DH and WS
This section discusses the yield of MCC extracted from different parts of the durian rind, i.e., the durian husk (DH) and the white sac (WS). It is apparent that each part yields a different amount of MCC. Based on the results shown in Table 5, with the same cellulose-to-acid ratio and acid concentration, the yield of MCC extracted from DH-30B (72.59%) is higher than that of WS-30B (66.10%). These yield values are lower than that reported by Baruah et al. [55] of 83%, where the study used sulfuric acid. However, the results obtained in the present work are acceptable because, in most of the investigations, the MCC yield does not affect the crystallinity index [56].
The higher yield of MCC extracted from DH indicates that its cellulose has more crystalline regions compared to WS. DH experiences faster hydrolysis during acid hydrolysis due to its higher dry content, as mentioned in Section III.A and non-cellulosic compounds, producing higher crystalline regions. Moreover, extracting MCC from cellulosic materials through acid hydrolysis breaks down the β-1,4 glycosidic linkages that bind the glucose monomers in cellulose and cause the cellulose molecules to hydrolyze [57]. Hydronium ions infiltrate the cellulosic material during hydrolysis, primarily disrupting the non-crystalline areas around the microfibrils and those embedded between them. By targeting the amorphous domains, the hydrolysis process can achieve faster reaction kinetics, resulting in a swift reduction of the degree of polymerization while maintaining the crystalline area untouched. When the well-structured crystalline regions achieve the maximal hydrolysis yield, further depolymerization will impede because the hydronium ions find it challenging to reach the crystalline region [58].
In summary, the cellulose yield and MCC yield were affected by various factors. Figure 8 summarizes the cellulose and MCC yield based on different conditions and parameters during the extraction process. The yield of cellulose was notably affected by the bleaching agent used. Sodium hypochlorite (NaClO) produces lower cellulose yield than sodium chloride and acetic acid. Additionally, the acid concentration and cellulose-to-acid ratio were major factors affecting the MCC yield during acid hydrolysis. Figure 8(b) and (c) demonstrated that a lower acid concentration of 30% (w/v) and a lower cellulose-to-acid ratio of 1:10 resulted in a much higher MCC yield of 72.59%. Furthermore, the results show that the yield of MCC extracted from WS is lower than that from the DH under similar acid concentrations and cellulose-to-acid ratio.
Therefore, the optimal parameters and conditions for extracting MCC from DH and WS at higher yields are an acid concentration of 30% (w/v), a cellulose-to-acid ratio of 1:10, and the use of NaClO as a bleaching agent. However, it is important to note that the ideal parameters for MCC extraction may differ based on the raw material utilized and the specific extraction process conditions.
Functional Group in Extracted MCC
Figure 9 represents the FTIR spectra obtained for both untreated and MCC samples extracted from DH and WS using different concentrations of oxalic acid [30, 50, and 65% (w/v)]. The spectra revealed peaks at 3340 cm−1 and 3441 cm−1, indicating the presence of -OH groups, the predominant functional groups in cellulose [59; 60]. The intensity of these peaks deepened after acid hydrolysis and pretreatment, suggesting an increase in the hydrophilic characteristics of cellulose, primarily due to a higher concentration of hydroxyl groups in the fibers, as reported by Fitriani et al. [26].
The absorption bands observed at 2990 cm−1 to 2882 cm−1 represent the symmetric and asymmetric vibrations of C-H bonds [61]. The sharper peaks in the MCC samples indicate enhanced exposure of the cellulose components. The band corresponding to hemicellulose is visible between 1765 cm−1 and 1715 cm−1, attributed to the C = O stretching of carbonyl (ketone) groups [62]. The absence of peaks in the MCC samples, except for the DH-30B and WS-30B at 1734 cm−1 (Fig. 9b), can be attributed to the removal of hemicellulose through pretreatment. The sharp peak at 1734 cm−1 in DH-30B represents acetyl and uronic ester groups in hemicellulose or the ester linkage of the carboxylic group in ferulic and p-coumaric in lignin and/or C = O group in hemicellulose [63]. This indicates that the complex structure of the biomass can affect the effectiveness of hemicellulose removal even under standardized conditions and parameters during alkaline treatment.
Additional bands detected in the FTIR spectrum, specifically at 1632 cm−1 for all samples (untreated and MCC), resulted from H-O-H bonding as the fibers absorb water. Despite undergoing a suitable drying procedure, removing water from the fibers proved challenging [6; 64]. Recent studies reported by Akhavan-Kharazian and Izadi-Vasafi [65] on the nanocellulose also proved the occurrence of hydrogen bonding in cellulose. The peaks observed in the FTIR spectrum, ranging from 1405 cm−1 to 895 cm−1 are indicative of cellulose presence and were detected in MCC samples but not significantly in untreated samples. Previous studies reported by Fazeli et al. [66] have shown that the peak indicates the presence of the CH2 group in cellulose at 1405 cm−1. In all MCC samples, peaks were observed at 1368 cm−1 due to the twisting vibration of C = O and C-H bonds [67], and the band at around 1313 cm−1 represents the CH2 wagging vibration within cellulose [45].
Furthermore, the visible peak at approximately 1157 cm−1 signifies the presence of glycosidic bonds within the cellulose chain. The absorption peak between 1027 cm−1 represents the vibration of the C-O-C bond, a bond found in cellulose [6]. The peak at 895 cm−1 signifies the C-O-C bonds within the β-1,4-glycosidic linkages of cellulose, as noted by Kusmono et al. [68].
The narrow absorption range between 1423 cm−1 to 890 cm−1 demonstrates that the cellulose structure of MCC samples comprises a more ordered structure than a disordered structure. This finding provides evidence that a high yield of MCC is being generated [69].
In summary, the FTIR results suggest that the cellulose structure remained intact after alkaline and bleaching treatment. The presence of hemicellulose peaks in the treated samples indicate that removing hemicellulose is challenging due to its complex structure. The absence of lignin peaks confirms the successful removal of lignin. Overall, the FTIR peaks support the successful cellulose extraction and suggest that further research should be conducted on the alkaline treatment of durian husk to explore hemicellulose removal.
Crystallinity Index of Extracted MCC
X-ray diffraction was employed to investigate the crystalline properties of cellulose treated with different acid concentrations and bleaching agents. The comparison of the XRD spectra for treated cellulose is presented in Fig. 10. The sharp and broad peak ranging from 22° to 24° suggests that all the extracted MCC exhibit a high degree of crystallinity. These results are similar to the work reported by Sainorudin et al. [70] on the MCC extracted from coconut coir, banana stem, sugarcane bagasse, and pineapple leaves. In the present work, DH-30B and WS-30B, which were treated with sodium hypochlorite, exhibited three peaks around 2θ = 16°, 22°, and 34°, corresponding to the (110), (200), and (004) diffraction planes. These peaks represent the typical crystalline structure of cellulose I [71]. Moreover, DH-30 A, DH-50 A, and DH-65 A, which were treated with sodium chloride and acetic acid, displayed three well-defined peaks around 2θ = 12°, 20° and 22°, corresponding to the planes of (1\(\:\stackrel{-}{1}\)0), (110) and (020). These three peaks are attributed to the antiparallel structure of cellulose II [72]. It is noteworthy that cellulose I and cellulose II are two different types of cellulose. The differences in XRD patterns between those samples indicate variances in cellulose structure due to the different bleaching agents employed.
Table 6 tabulates the calculated crystallinity (Crl) using Eq. (4) with the intensity of cellulose I and cellulose II at 2θ between 21° and 22°, and the intensity of amorphous region (Iam) at 2θ between 16° to 18°. The crystallinity for all MCC samples ranges from 71.43 to 78.30%. In the batch treated with sodium hypochlorite, DH-30B has a higher crystallinity index of 77.54% compared to WS-30B (73.81%). This may be due to the higher content of dry matter such as protein, starch, hemicellulose, lignin, and cell wall material in DH-30B (Section III.A), which gives a higher rate of hydrolysis than in WS-30B. The hydrolysis process increases the crystallinity index of DH-30B by breaking down the amorphous region, leaving behind a higher proportion of crystalline parts [73].
The calculated crystallinity index is qualitatively similar to Nasution and Sitompul [74], in which MCC was extracted from the fiber of empty fruit bunch palm oil and gave a crystallinity index of 73%. Variations in cellulose characteristics derived from different sources can lead to differences in the crystallinity index. The parameters used for the acid hydrolysis process can also influence the MCC properties.
For the batch treated with sodium chloride and glacial acetic acid (DH-30 A, DH-50 A, and DH-65 A), the crystallinity decreased from 78.3 to 71.43% as the acid concentration increased from 30 to 65% (w/v). This is because cellulose consists of both amorphous and crystalline components, and the amorphous region is more susceptible to hydrolysis [75]. The amorphous region of cellulose can be easily broken down by a lower acid concentration of 30% (w/v) without further degradation of sugar, resulting in smaller-sized cellulose with a greater crystallinity index. However, the crystalline region may suffer damage when a higher acid concentration, such as 65% (w/v), is employed, leading to a decrease in the crystallinity index [76].
Based on the X-ray diffraction analysis of MCC treated with different bleaching agents and acid concentrations, the study showed the various peaks in the XRD spectra indicated different cellulose structures, with NaClO-treated samples producing cellulose I, while NaOH and acetic acid-treated samples exhibiting cellulose II. On the other hand, higher acid concentration resulted in lower crystallinity due to damage to the crystalline region, although the overall crystallinity differences were not significant.
Morphology and Particle Size of Extracted MCC
Figure 11 illustrates the UV-Vis spectra of MCC in distilled water. In general, the transmittance of DH-30B (Fig. 11a) is higher compared to that of WS-30B (Fig. 11b). DH-30B achieved the highest transmittance value of 90% at around 300 nm, which could be attributed to the variations in particle size between DH-30B and WS-30B (Fig. 12e and f). The variations in transmittance due to differences in particle size were supported by previous research. Zhai et al. [77] found that the large-sized particle original fiber has lower transmittance of 92.8% compared to the homogeneous fiber suspension with a transmittance of 96.7%. Figure 11 shows that there is a slight decline at approximately 250 nm to 300 nm wavelength and this could be translated to increased interference in the UV range. Previous research on polymer/CNWs composite films reported that the agglomerations of CNWs might result in interference in the lower spectrum, which reduces the UV light transmittance [78]. Hence, areas with high MCC density in distilled water may resemble the agglomerated areas in polymer/CNWs composite films, producing an interference effect if the MCC and distilled water do not mix homogeneously. Furthermore, another study has suggested that nanoscale fillers with less than 25 nm sizes may prevent scattering and maintain the material’s transparency, resulting in high transmittance [79]. Since this research focuses on micro-sized crystalline cellulose, the slight decrease shown in the 250 to 300 nm wavelength region could be explained by bigger particle sizes (> 25 nm), which is consistent with the results that show the influence of particle size on transmittance. According to Handoko and Yusuf [79], the transmittance is influenced by the dispersion of the particle, including the presence of agglomerations. The percentage of transmittance is wavelength-dependent, and more light scattering occurs when the wavelength of light is either similar to or smaller than the diameter of the particle. Therefore, in the present work, it can be speculated that the lower transmittance of WS-30B indicates the presence of agglomerations in the extracted MCC, thus forming a barrier to the passage of light. Cellulose in crystal form has a propensity to interact with each other due to their increased surface area of reaction, leading to a greater likelihood of aggregation [80].
The average particle sizes and size distribution obtained through laser scattering for all MCC samples are listed in Table 7. It is crucial to note that the size distribution does not indicate the width or length of the particles; instead, it provides an apparent “size” for all particles, including individual particles and agglomerated or aggregated particles [81]. Compared to DH-50 A and DH-65 A, DH-30 A has the smallest average diameter, measuring 6.237 μm with a size distribution of 0.4178 μm. This finding parallels to El-Sakhawy and Hassan [82], in which the MCC is extracted from different agricultural residues with the average particle size ranging between 4.06 and 5.49 μm.
Meanwhile, DH-50 A and DH-65 A have average diameters of 25.38 μm and 19.04 μm, with size distributions of 0.002334 μm and 0.2208 μm, respectively. This is associated with an increase in oxalic acid concentration from 30% (w/v) to 65% (w/v), which resulted in a suspension with increasing particle size [83], indicating that more amorphous regions are being removed with lower acid concentration during acid hydrolysis. These results confirm that oxalic acid concentration is one of the most important factors dictating the reaction. Other researcher [84], also found that smaller particle sizes could be produced with lower acid concentrations. Sriruangrungkamol et al. [85] extracted nanocellulose from cellulose through varying acid concentrations and reported that the smallest particle size (31 ± 12 nm) was obtained with 5% (w/v) oxalic acid concentration, while 10% oxalic acid produced the largest nanocellulose with a particle size of 91 ± 33 nm. Therefore, in the present study, an oxalic acid concentration above 30% (w/v) favors acid hydrolysis. In general, excessive acid concentration at 65% (w/v) can damage the crystal structure of fibers and may even cause the fibers to carbonize, resulting in smaller particle sizes. However, since oxalic acid is a weak acid, it requires a higher temperature during hydrolysis [24] to produce smaller particle sizes efficiently. In contrast, acid hydrolysis using sulfuric acid typically operates at lower temperatures. Therefore, a low concentration of 30% (w/v) might be suitable for small MCC particle extraction with a temperature of 90 ℃. In contrast, higher oxalic acid concentrations, such as 50% (w/v) and 65% (w/v), may require a higher temperature to produce smaller MCC particles.
Moreover, the significant variance in individual particle diameter and size distribution for the extracted MCC is due to larger agglomerated and aggregated particles coexisting with individual microcellulose particles in the extracted MCC. This discrepancy suggests that different particle sizes due to aggregation and agglomeration cause inconsistencies in particle size.
In comparison between the MCC particle size extracted from different parts of the durian husk using the same acid concentration of 30% (w/v), DH-30B has a smaller average particle size (8.215 μm) than that of WS-30B (15.10 μm). This is in line with the UV-vis spectra (Fig. 11) that indicate a higher transmittance in DH-30B than in WS-30B, suggesting that UV-vis can be used to correlate the particle size of MCC through the turbidity of the suspension. The small particle size in DH-30B explains that the MCC extracted from different parts of the durian husk (DH and WS) affects the particle size, shape, and agglomeration characteristics. Bhardwaj et al. [86] stated that the removal of non-cellulosic compounds such as lignin, hemicellulose, and other impurities through pretreatment results in substantial morphological alterations in their size dimensions and structure.
Relating the results from particle size analysis to the previous section (Section III.A), DH-30B has more impurities than WS-30B, including protein, starch, and cell wall materials. Hence, more impurities are being removed from DH-30B through pretreatment, significantly reducing its particle size.
MCC extracted through different pretreatment and acid hydrolysis conditions were examined under FESEM, revealing that the extracted MCC has an irregular and micro-sized rod shape with a slightly rough surface (Fig. 12). This is attributed to the breakdown of cellulose fibers during the acid hydrolysis process. The uneven surface of the extracted MCC results from the cleavage of cellulose’s amorphous areas, breaking the fibers into shorter lengths and producing considerably weaker interactions that form cracks on the surface [87].
According to Kian et al. [88], MCC exhibits a diameter ranging from 40 to 300 μm. Particle size distributions measured through laser scattering (Table 7) proved that the extracted MCC are in the range of micro-sizes. The diameter of the MCC was further analyzed using ImageJ software to confirm the successful extraction of micro-sized cellulose from DH and WS. DH-30 A sample has a particle diameter ranging from 8.117 μm to 50.635 μm (Fig. 12a); however, the average particle size in Table 7 is approximately 6.237 μm. The SEM image of DH-30 A under 300X magnification (Fig. 12a) primarily shows bigger particles, while under the magnification of 50,000X (Fig. 12b), it reveals numerous smaller particles ranging from 0.164 μm to 0.324 μm. These explain why the average particle size of 6.237 ± 2.975E-2 μm measured using laser scattering is not within the range between 8.117 μm and 50.635 μm, which was measured using image J. Smaller particles were not observed in Fig. 12a as they may hide behind the bigger particles. The particle diameter of DH-50 A (Fig. 12c) also exhibits in micro sizes, ranging from 7.734 μm to 31.722 μm. However, DH-65 A (Fig. 12d) differs from those observed in DH-30 A (Fig. 12a and b) and DH-50 A (Fig. 12c), exhibiting an irregular, clear, smooth, and nonporous surface and a smaller particle size range between 6.425 μm and 28.619 μm. This could be attributed to the non-cellulosic components around the fibers, such as hemicellulose and lignin, that dissolved in the chemical during the acid hydrolysis [89]. A similar result was reported by Zhao et al. [90], in which MCC was extracted from tea waste, and the results show that the MCC detected consisted of individualized, long smooth fibers due to the acid penetration into the amorphous areas during hydrolysis leading to the cleavage of the β-1,4-glucopyranose linkage within the cellulose.
For samples used to study the effect of MCC extracted from different parts of the durian husk, DH-30B (Fig. 12e) and WS-30B (Fig. 12f) appear as aggregated cellulose micro balls, with some particles having larger sizes and irregular, rough surfaces. This contrast with the micro-sized rod-like structure observed in DH-30 A, DH-50 A, and DH-65 A (Fig. 12a-d) could be attributed to the presence of non-cellulosic compounds, such as hemicellulose, identified through FTIR analysis in Section III.F. Tan et al. [91] and Adawiyah and Suryanti [92] similarly noted that MCC extracted from lembang (Grewia florida) and cotton waste via acid hydrolysis having long threads-like structure, rod-like particle, in contrast to the micro-ball morphology, and uniform particle size. DH-30B (Fig. 12e) appears as fragments of microfibers with particle sizes between 5.201 μm and 24.849 μm, while WS-30B (Fig. 12f) exhibits larger particle size, ranging between 7.892 μm and 24.957 μm. Notably, this matches the findings from UV-vis (Fig. 11) that the DH sample shows higher transmittance in suspension, speculating a smaller particle size.
Mechanical Properties of Biocomposites
MCC has been identified as an effective reinforcing agent that enhances the mechanical properties of the polymer matrix. Figure 13 reveals that the tensile strength changes when untreated durian husk powder and MCC are added to polycaprolactone (PCL). The tensile strengths increased when 0.2 wt% of untreated durian husk powder (DH/untreated and WS/untreated) and MCC extracted from DH (DH/MCC) was added to PCL (Fig. 13a). The tensile strength of WS/untreated further increases when the concentration is raised to 0.5 wt% (Fig. 13b) and begins to decrease at a concentration of 1.0 wt% (Fig. 13c). However, the tensile strength of DH/untreated and DH/MCC decreases at a concentration of 0.5 wt% and increases at a concentration of 1.0 wt%.
In contrast, WS/MCC shows a different trend than the other samples. WS/MCC0.2 (with 0.2 wt% MCC) has lower tensile strength compared to pure PCL, and it starts to decrease as the concentration of MCC increases (Fig. 13d). The biocomposite fabrication technique is the main cause of the decrease in tensile strength. According to Tharazi et al. [93], heating time and pressure affect the tensile strength of the biocomposite. Tensile strength increases when the heating time increases from 5 to 8 min. However, tensile strength decreases when the heating time exceeds 8 min. Additionally, high pressure can lead to low tensile strength because the pressure can potentially harm the matrix and result in inadequate matrix infiltration. As a result, in the present work, the decrease in tensile strength of WS/MCC reinforced biocomposite could be the inconsistency in controlling the heating time and pressure during the fabrication. PCL was melted in a water bath at 70 ℃ throughout the fabrication of the biocomposite process. The heating time required to melt the PCL was not precisely measured, resulting in some samples being heated longer than others. In addition, the PCL was manually pressed after being filled into the mold. As a result, several disturbances were introduced to the fabricated biocomposites. Additionally, the cooling rate is another factor that impacts the mechanical properties of the biocomposites. Hence, future work should carefully manage the pressure, cooling and heating time during the biocomposite fabrication to ensure that fibers or MCC are distributed evenly in the matrix.
Overall, MCC extracted from DH has the potential to enhance the tensile strength of the PCL-based biocomposite, whereas MCC extracted from WS does not exhibit the same improvement. When 1.0 wt% of DH/MCC was added to the biocomposite, the tensile strength improved from 1.923 MPa to 2.339 MPa, representing a 22% increase. This finding aligns with the results reported by Van Hai et al. [94], indicating that crystalline cellulose can indeed enhance the tensile strength of the biocomposite. However, it is crucial to note that a large amount of crystalline cellulose can impact the interaction between the polymer matrix and reinforcing agent due to inadequate particle dispersion and the formation of large agglomerates. Consequently, the tensile strength decreases at high concentrations of MCC. Although DH/MCC and WS/MCC biocomposites were incorporated with microcrystalline cellulose, WS/MCC has a lower tensile strength. This could be due to the higher crystallinity of DH/MCC biocomposite compared to that of the WS/MCC. Faisal et al. [95] stated that the mechanical properties of the biocomposite are impacted by the crystallinity of the reinforcing agent, with higher crystallinity improving the mechanical properties. Other than that, the mechanical properties are also affected by the larger particle size caused by the agglomeration of the hydroxyl group on the WS surface, as indicated by UV-vis and FESEM analysis in Section III.H. Similar findings were reported by Luddee et al. [96], who found that the tensile strength and elongation of the polylactic acid (PLA) incorporated bacterial cellulose composite significantly decreased when the bacterial cellulose particle size increased from 90 to 180 μm. According to Lu et al. [97], the enhancement in the mechanical properties of the biocomposite could be attributed to the hydrogen bonding between the hydroxyl group of the filler and the polymer matrix molecules, strengthening the interfacial interaction. In the present work, WS/MCC exhibited a lower tensile strength because the hydroxyl groups on the WS surface attempt to form strong hydrogen bonds with adjacent hydroxyl groups in the WS chain instead of forming strong bonds with the PCL polymer. The strong hydrogen bonds on surface lead to entanglements and agglomerations of the nanocellulose, resulting in lower tensile strength.
The untreated durian rind also has a larger particle size since the non-cellulosic compounds are not being removed. Therefore, a noticeable gap between the matrix and fibers was created, which implies a weak interaction between the matrix and the fibers, as shown in Fig. 14a. According to Huang et al. [98], the mechanical properties of the matrix can be enhanced by adding fibers. The fibers bear the majority of the tensile load applied on a sample and bridge cracks in the matrix, thereby slowing the growth of cracks by dissipating energy close to the crack tip. Hence, the gap between the untreated durian rind and PCL indicates the poor compatibility of the fiber-matrix bonding, resulting in lower tensile strength and Young’s modulus as the tension load required to break the fiber-matrix bond is minimal.
The effect of reinforcing MCC and untreated durian husk on Young’s Modulus of the biocomposite was further explored in this study, and the results are illustrated in Fig. 15. Young’s Modulus decreases when untreated durian husk powder and MCC are added to the PCL. Notably, DH/MCC0.2 exhibits the lowest Young’s Modulus at 237.652 MPa, but it increases to 357.784 MPa when the MCC concentration reaches 1.0 wt%. The result is in agreement with an earlier work reported by Rose Joseph et al. [99], Young’s Modulus tends to increase with the addition of reinforcing agents. Moreover, Paul et al. [100] also found similar results where the Young’s Modulus increased from 1556 MPa to 2517 MPa when MCC was added to PLA. The strong compatibility of natural filler with the polymer matrix, facilitated by hydrogen bonding interactions, enhances the efficient stress transfer from the PCL polymer matrix to MCC, resulting in improved mechanical properties. However, in the present work, the Young’s Modulus of DH/untreated, WS/untreated, and WS/MCC remains lower than that of the pure PCL. A low Young’s Modulus in these biocomposites suggests weak intermolecular interactions between the reinforcing agent (untreated powder and MCC) and PCL [101]. Additionally, the decrease in Young’s modulus is attributable to the aggregation of the reinforcing agent at higher concentrations, driven by the large specific area and high surface energy of the particles in the filler. Hence, a higher concentration of reinforcing agent is likely to cause agglomerations, which lowers and reduces the mechanical properties. Besides that, during the fabrication of biocomposites, a concurrent and reversible agglomeration process occurs alongside the aggregation [102]. Furthermore, the crystallinity index of the reinforcing agent (Section III.G) also impacts the mechanical properties. The more ordered structure of DH/MCC compared to the untreated fibers and WS/MCC likely contributes to the superior mechanical properties of the DH/MCC, hence the biocomposites [103].
Moreover, the influence of additional MCC and untreated durian husk on the elongation at break of the biocomposite is depicted in Fig. 16. It can be clearly seen that the percentage of elongation at break fluctuates as the concentration of MCC and untreated durian husk increases, with the minimum and maximum elongation at break recorded at 0.557% and 0.934%, respectively. All samples exhibit an increase at the 0.2 wt% concentration of untreated durian husk and MCC, except for WS/MCC, suggesting that incorporating MCC and untreated durian husk at 0.2 wt% enhances ductility. The improved distribution of MCC in the PCL matrix contributes to the increased ductility at the 0.2 wt% concentration. However, this does not necessarily imply greater mobility of the PCL chain; it simply results in increased ductility. Furthermore, the addition of 0.5 wt% MCC reduces the ductility of the biocomposite, likely caused by the restricted movement of the PCL chain due to the introduction of a larger quantity of MCC [68].
When the concentration is increased further to 1.0 wt%, the elongation at break increases again, except for WS/MCC (Fig. 16d). Other researchers have also demonstrated a decrease in elongation at break with the increasing concentration of the natural reinforcing agent. Sultana et al. [104] found that the elongation at break decreased with the increasing content of nanocellulose and cellulose from 1 to 5 wt% in PVA biocomposites. Similar findings were also reported by Noorbakhsh-Soltani et al. [105], where the maximum values in elongation at break were achieved for 5 wt% natural filler. However, the elongation at break decreased further as the filler concentration increased to 25 wt%. The low elongation at break is attributed to the brittle and rigid behavior of crystalline cellulose. Mohan Bhasney et al. [11] also found that adding MCC to PLA reduced the tensile strength while increasing Young’s Modulus. In comparison to pure PLA, the tensile strength and percentage elongation decreased to about 32 MPa and 10.3%, respectively, upon incorporating 0.1 wt% MCC. When the MCC concentration increased to 0.5 wt%, the percentage elongation and tensile strength decreased by approximately 34% and 54%, respectively. Hence, in the present work, the elongation at break is also affected by the amount of reinforcement added, the dispersion of the reinforcement within the PCL, and the interaction between the matrix and the reinforcement [106]. MCC tends to agglomerate, resulting in poor dispersion and interaction in the PCL matrix. As a result, the elongation at break decreases.
Table 8 summarizes the tensile strength, Young’s Modulus, and percent of elongation for PCL-based composite. The MCC extracted from DH has shown promising potential as a natural reinforcing agent to improve the mechanical strength of biocomposite. This improvement is consistent with earlier research showing that crystalline cellulose, such as MCC, can significantly increase the tensile strength of polymer matrix. The present study also showed that the MCC extracted from DH tends to increase the Young’s Modulus compared to pure PCL biopolymer, indicating improved stress transfer within the PCL-based biocomposite. Though this work has shown enhancement of mechanical properties, further study is recommended to improve the mechanical properties of PCL-based composites. One promising approach involves modifying crystalline cellulose to enhance its interface compatibility with the polymer matrix. For instance, Jin et al. [107] found that the compatibility of crystalline cellulose and polylactic acid (PLA) can be enhanced through surface modification because the alkyl side chains grafted onto the crystalline cellulose surface and the PLA side chain intertwined and interpenetrated, increasing the intermolecular interaction force and the effective contact area between the modified crystalline cellulose and PLA. Huang et al. [108] also performed surface modification to increase the hydrophobicity and dispersibility of crystalline cellulose using stearic acid. The modified crystalline cellulose was incorporated into PLA film-forming at different concentrations. The modified crystalline cellulose forms a stable network with PLA by entanglement and co-crystallization of the molecular chains. This interaction strengthened the bonding between the reinforcing agent and polymer matrix, leading to higher elastic modulus and tensile strength.
Analysis of Fracture Surfaces of PCL/MCC Biocomposite
The SEM images of the fracture surfaces after tensile testing with fiber pullout, some voids, waviness, and fiber fracture for WDH/Untreated and MCC-reinforced PCL composites (MCC/WPO) are shown in Fig. 14. Fig. 14a-d show the PCL incorporated with untreated DH and WS, while Fig. 14e-h illustrated the PCL incorporated with MCC extracted from DH and WS. The presence of small void space can be observed in all samples. It is clear that fiber pullouts are not the main cause of this small void space. These void spaces, also known as gas porosities [109], are likely attributed to the shrinkage of the sample from molten to solid state during the biocomposite fabrication process. On the other hand, the void space within the biocomposite also indicates that the MCCs are not evenly distributed [110].
The SEM images depicted that the fracture parts have a rough surface due to the addition of untreated durian rind and MCC. The untreated fibers are dispersed throughout the PCL matrix in DH/untreated biocomposite (Fig. 14a). However, the MCC in DH/MCC1.0 cannot be observed due to the small micro-sized MCC. The PCL biocomposite incorporated with DH/untreated1.0 exhibited an agglomeration and large porosity on its surface (Fig. 14a). However, there are still noticeable gaps between the matrix and fibers (indicated by arrow), which implies a weak interaction between the matrix and the fibers [35].
Compared to DH/MCC1.0 and WS/MCC1.0, the mechanism of fiber pullout is more noticeable in DH/untreated1.0 and WS/untreated1.0. This could be attributed to fiber debonding during fracture, leading to an extended crack propagation length during tensile testing and, consequently, an enhancement in fracture toughness [111]. According to Pavan et al. [112], when the particle is pulled and slid over the PCL matrix, a tiny peak will be created at the location where the particle is ejected. Smaller particles like MCC are easily removed, whereas large particles like untreated durian husk can withstand the load and leave an imprint before being pulled out from the surface.
Biodegradability Test
Research on biodegradation characteristics is crucial for understanding their degradation process and promoting sustainability. This study conducted soil burial experiments for pure PCL and the PCL-based biocomposites containing 0.2 wt%, 0.5 wt%, and 1.0 wt% of MCC extracted from DH and WS. The percentage of weight loss for each biocomposite over biodegradation time is plotted in Fig. 17. After 7 days of incubation in soil, only pure PCL and WS/MCC (0.2, 0.5, and 1.0 wt%) experienced weight losses, ranging between 0.07 and 0.15%. By day 28, the results revealed that the weight losses of the WS/MCC biocomposites were all higher than that of pure PCL. Therefore, the presence of MCC in biocomposites increased the degradation of PCL as these biocomposites exhibit more significant weight loss than that of pure PCL, as evidenced by the steeper percentage weight loss curve in Fig. 17.
When comparing the weight loss of WS/MCC with DH/MCC biocomposites at 0.2, 0.5, and 1.0 wt.% MCC concentrations, WS/MCC demonstrates a higher weight loss percentage than DH/MCC. This is because cellulose, which is a molecule consisting of two regions, namely highly oriented molecules known as ‘crystalline cellulose’ and less oriented molecules called ‘amorphous cellulose’ [113], can significantly impact the degradation ability based on its physical and chemical properties, including morphology, orientation, and crystallinity. Research indicates that highly crystalline cellulose is more challenging to degrade. The XRD analysis shows that DH-30B has a higher crystallinity index than WS-30B (Table 6); hence, DH/MCC biocomposite exhibits higher crystallinity than WS/MCC biocomposite, making it more resistant to microorganism attacks [114]. Higher crystallinity in DH/MCC indicates that the structure of DH is highly ordered and tightly packed compared to WS, which has lower crystallinity, is less ordered, and is more accessible to microorganisms [115]. This explains the variations in weight loss between DH/MCC and WS/MCC.
However, the negative weight loss observed in the DH/MCC at 0.2, 0.5, and 1.0 wt% MCC concentrations indicates a weight increase upon incubation in soil. Durian husk, containing natural fibers, exhibits hydrophilic properties [114] and can easily absorb moisture [116]; resulting in the weight increase. Research suggests that moisture can enter biocomposites through three main mechanisms [94]. Firstly, water molecules diffuse into the tiny gaps between polymer chains. Secondly, capillary transport occurs through openings and imperfections resulting from inadequate wettability between the matrix and fibers. Thirdly, the transfer happens via microcracks that develop in the matrix due to fiber swelling or during the mixing process with the fiber [117]. Therefore, the hydrophilic properties of natural fibers can affect the weight loss performance of biocomposites.
In contrast, WS/MCC showed a positive weight loss, probably due to the degradation caused by microorganisms. In a wet environment, water molecules infiltrate through the microcracks caused by fiber swelling during water absorption, reducing the interfacial adhesion between the polymer matrix and fiber [118]. This facilitates the transport of water containing microorganisms and their enzymes within the polymer matrix [119; 120], thereby enhancing the biodegradability of the WS/MCC biocomposites.
As illustrated in Fig. 17, the biocomposites demonstrated a degradation rate of approximately 0.07% after 7 days and increased to 0.42% over 28 days. The considerably low degradation rate may be attributed to the hydrophilic properties of the MCC, which increased water uptake in the biocomposites, thereby preventing a reduction in weight loss. Additionally, due to time constraints, our study only covered a 28-day period, which may not have been sufficient for observing a more significant degradation of the biocomposite. Other studies [121] have conducted the biodegradability test over more extended period and observed that biocomposite degrades more quickly over longer times, such as 5 months. However, the initial degradation rate was very slow, consistent with our findings. The low degradation rate in our study could also be due to the type of soil used, which is natural soil. Other researchers have found that the weight of biocomposite decreases more rapidly in compost soil than in natural soil due to the high humidity of compost soil and neutral pH of 7, which is an optimal condition for microorganism growth [122].
In summary, the biodegradability results clearly indicate that PCL/MCC biocomposites do not incorporate any adverse ecological effects. In other words, PCL/MCC biocomposites are entirely biodegradable.
Thermal Stability
TGA was performed to examine the thermal properties of the PCL-based biocomposite and monitor changes in weight%. This technique is helpful in identifying the maximum temperature that scaffolds can withstand during thermal processing and their homogeneity. Figures 18 and 19 shows the thermogravimetric (TG) thermogram and first-order derivative (DTG) of the PCL-based biocomposites. The onset, maximum, and endset degradation temperatures, determined through TGA analysis, are presented in Table 9. Virgin PCL degrades in a single step, beginning at 410.12 °C and ending at 462.38 °C (Table 9). On the other hand, the addition of untreated durian rind and MCC to PCL increased the onset degradation temperature to a range between 417.77 and 420.74 °C. The lower onset degradation temperature of virgin PCL suggests that it reached the exothermic peak faster than the PCL-incorporated natural reinforcing agent [123]. Ye et al. [124] also observed similar results, where the thermal stability of the PCL significantly improved with the addition of MCC, suggesting that adding MCC prevents PCL from degrading and enhances its thermal stability. Both virgin PCL and biocomposites displayed a single degradation curve in the TGA curve, indicating the excellent miscibility of the biocomposites [125].
In PCL/MCC biocomposites, the onset degradation temperature increased with higher MCC content. The thermal degradation temperature rises due to the increase in molecular weight caused by the cross-linking interaction between the PCL matrix and MCC or the molecular chain extension of the matrix itself [126]. Consistent with these findings, Abbas et al. [127] also noted that the enhanced thermal stability is attributed to the positive interactions between PCL and MCC. Hydrogen bonds formed between the hydroxyl groups of MCC and carbonyl groups of PCL prevent the fast dehydration of the MCC. Additionally, gases and char produced during cellulose breakdown interact with the solid PCL, further enhancing the thermal stability [128]. Thus, MCC serves as a stabilizer that accounts for the increased thermal stability. In contrast, the thermal stability of the biocomposites incorporated with untreated durian rind slightly decreases when the filler loading increases, mainly due to the interaction between the natural reinforcing agent and the PCL matrix. The inadequate interfacial adhesion between the hydrophobic PCL and hydrophilic fiber leads to poor compatibility between the two materials. As the concentration of untreated durian rind increases, the incompatibility increases, resulting in reduced thermal stability [129].
According to the TGA data, biocomposites with untreated durian rind have higher thermal stability than PCL/MCC biocomposites. The untreated durian rind consists of non-cellulosic compounds, such as hemicellulose (30.7%) and lignin (15.6%), which affect the thermal stability of the biocomposites. According to Gond et al. [130], treated fiber exhibits lower thermal stability than untreated fiber due to the elimination of protective hemicellulose and lignin, which serve as a thermal barrier. These findings demonstrate that the amount of lignin, hemicellulose, and cellulose in a biocomposite affects its initial decomposition temperature and thermal stability.
Another important thermal property of the biocomposite is the temperature corresponding to the maximum degradation rate of weight loss (Tmax). Tmax is identified as the peak found in the first derivative curve of the TGA thermograph. From Figs. 18 and 19; Table 9, it is evident that raising the untreated durian rind or MCC content in the PCL biocomposite increases the maximum degradation temperature, except for DH/untreated. The increased Tmax indicates that thermal stability improved due to the incorporation of a reinforcing agent as a barrier layer. These layers restrict the release of generated degradation gasses and heat transmission, thereby improving the thermal stability of biocomposites [131]. However, the increase in Tmax is insignificant, at around 2 to 3 °C only. Similarly, Onbattuvelli et al. [132] have found that degradation temperature is usually affected by increasing the reinforcing agent content in PCL-based composites. They noted that the degradation temperature might change with greater concentrations of crystalline cellulose, such as 11 wt%, mainly because the higher degradation temperature is attributed to the increased interfacial area between crystalline cellulose and polymer.
In summary, incorporating untreated durian rind and MCC as a reinforcing agent in PCL-based biocomposites improved the thermal stability of the biocomposites. In addition, increasing the reinforcing agent content increases the thermal stability of the biocomposites.
Conclusion
This work focuses on extracting microcrystalline cellulose (MCC) from durian husk and explores its potential application as a reinforcing agent within biopolymer matrix. Extraction of MCC from whole durian husk (DH) and white sac (WS) through acid hydrolysis using different concentrations of oxalic acid and varying cellulose-to-acid ratios was investigated. The particle sizes of extracted MCC ranged from 6.237 μm to 25.38 μm. Findings from this work address the existing knowledge gap, demonstrating that lower oxalic acid concentrations at 30% (w/v) and lower cellulose-to-acid ratio (1:10) provide a better MCC yield. The highest extracted MCC yield was 72.59% for extraction from DH and 66.10% for extraction from WS. It is suggested that further reducing the acid concentration can enhance the yield, as some researchers found that oxalic acid concentrations lower than 30% (w/v) could provide a higher MCC yield.
Additionally, FTIR confirmed the presence of cellulose in the extracted samples. However, remnants of hemicellulose were found in samples. Incomplete removal is potentially due to the complex structure of hemicellulose, posing difficulty for its complete elimination. UV-vis analysis indicated that the transmittance of the MCC extracted from different parts of the durian husk varied, with the lower transmittance between 250 nm and 300 nm due to considerably larger particle size and the presence of agglomeration blocking the light. Furthermore, XRD indicated that the crystallinity index of the extracted MCC ranges between 71.3 and 78.3%.
PCL-based biocomposites with varying MCC concentrations were investigated. The tensile testing demonstrated that reinforcing MCC extracted from DH can improve the mechanical properties of PCL-based biocomposite, with the tensile strength improving by 12% and the Young’s Modulus improving by 15% when 1 wt% of MCC was added to the biocomposite. Since the tensile strength and elongation of the biocomposite demonstrated improvement with the addition of 1 wt% MCC, further work on the maximum concentration of MCC in the biocomposite without compromising the mechanical properties of the biocomposite can be explored. The cross-section of the biocomposite fracture surface indicated the contribution of the MCC during the pullout. Furthermore, the biodegradability test tracked the weight of the PCL/MCC biocomposites, revealing a weight loss ranging between 0.58 and 0.35% after 28 days.
Overall, based on the current study, the optimal conditions for MCC extraction are an acid concentration of 30% (w/v) and a cellulose-to-acid ratio of 1:10. These parameters offer the highest MCC yield. Nevertheless, it is worth exploring the extraction yield by further reducing the acid concentrations. When considering different parts of the durian husk, DH provides better results than WS because of its ability to provide higher MCC yield, better UV-vis transmission, smaller particle size, higher crystallinity, and improved mechanical properties when incorporated into PCL. Moreover, sodium hypochlorite (NaClO) served as a better bleaching agent compared to sodium chloride (NaCl) and glacial acetic acid (CH3COOH) for extracting cellulose from durian husk as it gives a higher yield and brighter color cellulose. Finally, the thermal stability of PCL after incorporating untreated fiber and MCC was increased, and this was proved by the higher onset temperature obtained through TGA analysis.
Considering the fabricated biocomposite exhibits a brittle property, using a thicker mold made of stainless steel, which has lower malleability, is recommended. The aluminum mold used in this work is too thin and susceptible to scratching, resulting in an uneven surface for the fabricated biocomposite. Moreover, the biocomposite can be fabricated using a hot press machine, which can maintain a constant pressure applied to the biocomposite and melt PCL in a shorter duration than in a water bath. The optimal temperature, pressure, and pressing duration during the fabrication must be determined, as these parameters vary based on the chosen polymer matrix. Lastly, it is advisable to explore the impact on the mechanical properties of the biocomposite by incrementally increasing the MCC concentration in the PCL matrix. Modifications to crystalline cellulose are also recommended to investigate the interface compatibility with the polymer matrix and further impact on the mechanical properties of the biocomposites.
Data Availability
No datasets were generated or analysed during the current study.
References
Rajeh C, Saoud IP, Kharroubi S, Naalbandian S, Abiad MG (2021) Food loss and food waste recovery as animal feed: a systematic review. J Mater Cycles Waste Manage. https://doi.org/10.1007/s10163-020-01102-6
Rhodes CJ (2019) Solving the plastic problem: from cradle to grave, to reincarnation. Sci Prog. https://doi.org/10.1177/0036850419867204
Celentano A (2022) Plastic Pollution Effects on Pacific Marine Life. PlumX Metrics. https://digitalcommons.sacredheart.edu/acadfest/2022/all/88/. Accessed 7 July 2024
Tiller R, Nyman E (2018) Ocean plastics and the BBNJ treaty—is plastic frightening enough to insert itself into the BBNJ treaty, or do we need to wait for a treaty of its own? J Environ Stud Sci. https://doi.org/10.1007/s13412-018-0495-4
Suriyatem R, Auras RA, Rachtanapun P (2019) Utilization of carboxymethyl cellulose from durian rind agricultural waste to improve physical properties and stability of rice starch-based film. J Polym Environ. https://doi.org/10.1007/s10924-018-1343-z
Romadhon ZRF, Sanjaya IGM (2021) Development of Nanocomposite films with Durian Peel Nanocrystalline Cellulose. J Akademika Kimia. https://doi.org/10.22487/j24775185.2021.v10.i4.pp230-236
Husin M, Li AR, Ramli N, Romli AZ, Hakimi MI, Ilham Z (2017) Preparation and characterization of cellulose and microcrystalline cellulose isolated from waste Leucaena leucocephala seeds. Int J Adv Appl Sci. https://doi.org/10.21833/ijaas.2017.03.009
Hua Y, Harun S, Sajab M, Jahim J, Shah S (2020) Extraction of cellulose and microcrystalline cellulose from Kenaf. Jurnal Kejuruteraan. https://doi.org/10.17576/jkukm-2020-32(2)-04
Chua JY, Pen KM, Poi JV, Ooi KM, Yee KF (2023) Upcycling of biomass waste from durian industry for green and sustainable applications: an analysis review in the Malaysia context. Energy Nexus. https://doi.org/10.1016/j.nexus.2023.100203
Miao C, Hamad WY (2013) Cellulose reinforced polymer composites and nanocomposites: a critical review. Cellulose. https://doi.org/10.1007/s10570-013-0007-3
Mohan Bhasney S, Kumar A, Katiyar V (2020) Microcrystalline cellulose, polylactic acid and polypropylene biocomposites and its morphological, mechanical, thermal and rheological properties. Compos Part B: Eng. https://doi.org/10.1016/j.compositesb.2019.107717
Abu J, Abdulsalam S, Mohammed J (2022) Production and optimization of pigments and Binder in low-cost Emulsion House Paint using response surface methodology. Nigerian J Trop Eng. https://doi.org/10.59081/njte.16.1.003
Zhan YF, Hou XT, Fan LL, Du ZC, Ch’ng SE, Ng SM, Thepkaysone K, Hao EW, Deng JG (2021) Chemical constituents and pharmacological effects of durian shells in ASEAN countries: a review. Chin Herb Med. https://doi.org/10.1016/j.chmed.2021.10.001
Lubis R, Saragih S, Wirjosentono B, Eddyanto E (2018) Characterization of durian rinds fiber (Durio zubinthinus, murr) from North Sumatera. AIP Conf Proc. https://doi.org/10.1063/1.5082474
Research PM Durian Market Share, Size, Trends, Industry Analysis Report, By Product Type (Fresh, Processes); By Processing Technology; By End-Use; By Region; Segment Forecast, 2024–2032. Polaris (2024) https://www.polarismarketresearch.com/industry-analysis/durian-market. Accessed 2 May 2024
Zhao C, Cui X, Liu Y, Zhang R, He Y, Wang W, Chen C, Liu G (2017) Maximization of the methane production from durian shell during anaerobic digestion. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.03.184
Brinchi L, Cotana F, Fortunati E, Kenny JM (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2013.01.033
Dziuba R, Kucharska M, Madej-Kiełbik L, Sulak K, Wiśniewska-Wrona M (2021) Biopolymers and Biomaterials for Special Applications within the Context of the Circular Economy. Materials. https://doi.org/10.3390/ma14247704
Das A, Ringu T, Ghosh S, Pramanik N (2023) A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym Bull. https://doi.org/10.1007/s00289-022-04443-4
Xian X, Wang X, Zhu Y, Guo Y, Tian Y (2018) Effects of MCC content on the structure and performance of PLA/MCC biocomposites. J Polym Environ 26:3484–3492
Dogu B, Kaynak C (2016) Behavior of polylactide/microcrystalline cellulose biocomposites: effects of filler content and interfacial compatibilization. Cellulose 23:611–622
Pusparizkita YM, Hidayatullah AF, Anwar NF, Junaidi J, Sudarno S (2022) Effect of drying duration on the water content of durian peel waste for bio pellet. IOP Conf Ser: Earth Environ Sci. https://doi.org/10.1088/1755-1315/1098/1/012052
Rahman WA, Ismail SNAS, Rosli M (2016) Morphology and properties of durian cellulose nanofibres reinforced polyvinyl alcohol/starch based composite. AIP Conf Proc. https://doi.org/10.1063/1.4965053
Xu W, Grénman H, Liu J, Kronlund D, Li B, Backman P, Peltonen J, Willför S, Sundberg A, Xu C (2017) Mild oxalic-acid‐catalyzed hydrolysis as a novel approach to prepare cellulose nanocrystals. ChemNanoMat. https://doi.org/10.1002/cnma.201600347
Song K, Ji Y, Wang L, Wei Y, Yu Z (2018) A green and environmental benign method to extract cellulose nanocrystal by ball mill assisted solid acid hydrolysis. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.06.128
Fitriani F, Aprilia S, Arahman N, Bilad MR, Amin A, Huda N, Roslan J (2021) Isolation and characterization of nanocrystalline cellulose isolated from pineapple crown leaf fiber agricultural wastes using acid hydrolysis. Polymers. https://doi.org/10.3390/polym13234188
Beltramino F, Roncero MB, Vidal T, Torres AL, Valls C (2015) Increasing yield of nanocrystalline cellulose preparation process by a cellulase pretreatment. Bioresour Technol. https://doi.org/10.1016/j.biortech.2015.06.007
Li W, Wang R, Liu S (2011) Nanocrystalline cellulose prepared from softwood kraft pulp via ultrasonic-assisted acid hydrolysis. BioResources. https://doi.org/10.15376/biores.6.4.4271-4281
Csiszar E, Kalic P, Kobol A, de Paulo Ferreira E (2016) The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2016.01.028
Hanif Z, Jeon H, Tran TH, Jegal J, Park S-A, Kim S-M, Park J, Hwang SY, Oh DX (2018) Butanol-mediated oven-drying of nanocellulose with enhanced dehydration rate and aqueous re-dispersion. J Polym Res. https://doi.org/10.1007/s10965-017-1343-z
Kian LK, Jawaid M, Ariffin H, Karim Z (2018) Isolation and characterization of nanocrystalline cellulose from roselle-derived microcrystalline cellulose. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.03.065
Khili F, Borges J, Almeida PL, Boukherroub R, Omrani AD (2019) Extraction of cellulose nanocrystals with structure I and II and their applications for reduction of graphene oxide and nanocomposite elaboration. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0202-4
Bajpai PK, Ahmad F, Chaudhary V (2017) Processing and characterization of bio-composites. Handb Ecomater. https://doi.org/10.1007/978-3-319-48281-1_98-1
Rasheed M, Jawaid M, Parveez B (2021) Biodegradable composites from bamboo fibers - mechanical and morphological study. J Polym Environ. https://doi.org/10.21203/rs.3.rs-298057/v1
Juntaro J, Pommet M, Mantalaris A, Shaffer M, Bismarck A (2007) Nanocellulose enhanced interfaces in truly green unidirectional fibre reinforced composites. Compos Interfaces. https://doi.org/10.1163/156855407782106573
Hossain N, Chowdhury MA, Noman TI, Rana MM, Ali MH, Alruwais RS, Alam MS, Alamry KA, Aljabri MD, Rahman MM (2022) Synthesis and characterization of eco-friendly bio-composite from fenugreek as a natural resource. Polymers. https://doi.org/10.3390/polym14235141
Wan YZ, Luo H, He F, Liang H, Huang Y, Li XL (2009) Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos Sci Technol. https://doi.org/10.1016/j.compscitech.2009.02.024
Hejna A, Formela K, Saeb MR (2015) Processing, mechanical and thermal behavior assessments of polycaprolactone/agricultural wastes biocomposites. Ind Crops Prod 76:725–733
Pusparizkita YM, Hidayatullah AF, Anwar NF, Junaidi J, Sudarno S (2022) Effect of drying duration on the water content of durian peel waste for bio pellet. IOP Conference Series: Earth and Environmental Science, IOP Publishing, 1098:012052
Scalisi A, O’Connell MG (2021) Relationships between soluble solids and dry matter in the flesh of stone fruit at harvest. Analytica. https://doi.org/10.3390/analytica2010002
Nordin N, Shamsudin R, Azlan A, Ya’acob ME (2017) Dry matter, moisture, ash and crude fibre content in distinct segments of ‘Durian Kampung’husk. Int J Chem Mater Eng. https://doi.org/10.5281/zenodo.1314584
Safari A, Karimi K, Shafiei M (2017) Dilute alkali pretreatment of softwood pine: a biorefinery approach. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.03.030
Putri DA, Kurniyati Z (2016) Effect of sodium chloroacetate towards the synthesis of CMC (carboxymethyl cellulose) from durian (Durio zibethinus) peel cellulose. Int J Innovative Res Adv Eng (IJIRAE). https://doi.org/10.6084/M9.FIGSHARE.3504209
Xing H, Fei Y, Cheng J, Wang C, Zhang J, Niu C, Fu Q, Cheng J, Lu L (2022) Green preparation of Durian rind-based cellulose nanofiber and its application in aerogel. Molecules. https://doi.org/10.3390/molecules27196507
Penjumras P, Rahman RBA, Talib RA, Abdan K (2014) Extraction and characterization of cellulose from Durian Rind. Agric Agric Sci Proc. https://doi.org/10.1016/j.aaspro.2014.11.034
Veisi H (2007) Sodium hypochlorite (NaOCl). Synlett. https://doi.org/10.1055/s-2007-986658
David C, Fornasier R, Thiry P (1985) Utilization of waste cellulose. Appl Biochem Biotechnol. https://doi.org/10.1007/BF02798669
Hameed A, Dekhyl A, Alabdraba WS (2022) Removing the acid orange 12 azo dye from aqueous solution using sodium hypochlorite, a kinetic and thermodynamic study. IOP Conf Ser: Earth Environ Sci. https://doi.org/10.1088/1755-1315/961/1/012056
Abdel-Halim E (2012) An effective redox system for bleaching cotton cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2012.05.044
Su XM (2014) Method for bleaching wood by utilizing sodium chlorite and acetic acid. Google Patent. https://typeset.io/papers/method-for-bleaching-wood-by-utilizing-sodium-chlorite-and-2xqrwoqhik. Accessed 7 July 2024
Fan JS, Li YH (2012) Maximizing the yield of nanocrystalline cellulose from cotton pulp fiber. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2012.01.081
Tang Y, Yang S, Zhang N, Zhang J (2014) Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose. https://doi.org/10.1007/s10570-013-0158-2
Ren H, Shen J, Pei J, Wang Z, Peng Z, Fu S, Zheng Y (2019) Characteristic microcrystalline cellulose extracted by combined acid and enzyme hydrolysis of sweet sorghum. Cellulose. https://doi.org/10.1007/s10570-019-02712-6
Doan TKQ, Chiang KY (2022) Characteristics and kinetics study of spherical cellulose nanocrystal extracted from cotton cloth waste by acid hydrolysis. Sustain Environ Res. https://doi.org/10.1186/s42834-022-00136-9
Baruah J, Deka RC, Kalita E (2020) Greener production of microcrystalline cellulose (MCC) from Saccharum spontaneum (Kans grass): statistical optimization. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.03.158
Kulkarni SJ (2023) Feedstocks, synthesis, and characterization of cellulosic materials for Advanced applications with emphasis on Microcrystalline Cellulose (MCC). BioNanoScience. https://doi.org/10.1007/s12668-023-01080-7
Dussan KJ, Silva D, Moraes E, Arruda PV, Felipe M (2014) Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse. Chem Eng Trans. https://doi.org/10.3303/CET1438073
Hao J, Ye W, Gao C, Zhu M, Yang L, Liao R (2022) Experimental and molecular level analysis of natural ester delaying degradation of cellulose insulation polymer. High Voltage. https://doi.org/10.1049/hve2.12151
Nasution M, Gea S, Sumaiyah, Wirjosentono B, Karsono (2014) Preparation and characterization of nanocrystalline cellulose from sugar palm bunch (Arenga pinnata (Wurmb) Merr). Int J PharmTech Res 6:814–820
Saragih SW, Wirjosentono B, Meliana Y (2019) Extraction and characterization of cellulose from abaca pseudo stem (Musa textile). J Phys: Conf Ser. https://doi.org/10.1088/1742-6596/1232/1/012018
Ghazy MB, El-Hai FA, El-Zawawy WK, Owda ME (2017) Morphology and mechanical properties of nanocrystalline cellulose reinforced chitosan based nanocomposite. ResearchGate. https://www.researchgate.net/publication/313377381_Morphology_and_mechanical_properties_of_nanocrystalline_cellulose_reinforced_chitosan_based_nanocomposite. Accessed 7 July 2024
Lubis R, Wirjosentono B, Eddyanto E, Septevani A (2020) Influence of homogenization time to the physical properties of durian peel’s pulp from North Sumatera Indonesia. AIP Conf Proc. https://doi.org/10.1063/5.0017423
Sun X, Xu F, Sun R, Fowler P, Baird M (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res. https://doi.org/10.1016/j.carres.2004.10.022
Punnadiyil RK, Sreejith M, Purushothaman E (2016) Isolation of microcrystalline and nano cellulose from peanut shells. J Chem Pharm Sci 974:2115
Akhavan-Kharazian N, Izadi-Vasafi H (2019) Preparation and characterization of chitosan/gelatin/nanocrystalline cellulose/calcium peroxide films for potential wound dressing applications. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.04.159
Fazeli M, Florez JP, Simão RA (2019) Improvement in adhesion of cellulose fibers to the thermoplastic starch matrix by plasma treatment modification. Compos Part B: Eng. https://doi.org/10.1016/j.compositesb.2018.11.048
Trivedi AK, Kumar A, Gupta M (2023) Extraction of nanocellulose from wheat straw and its characterization. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2022.11.038
Kusmono, Wildan MW, Lubis FI (2021) Fabrication and characterization of chitosan/cellulose nanocrystal/glycerol bio-composite films. Polymers. https://doi.org/10.3390/polym13071096
Wijaya CJ, Saputra SN, Soetaredjo FE, Putro JN, Lin CX, Kurniawan A, Ju Y-H, Ismadji S (2017) Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2017.08.004
Sainorudin MH, Mohammad M, Kadir NHA, Abdullah NA, Yaakob Z (2018) Characterization of several microcrystalline cellulose (Mcc)-based agricultural wastes via x-ray diffraction method. Solid State Phenom. https://doi.org/10.4028/www.scientific.net/SSP.280.340
Nindiyasari F, Erika G, Zimmermann T, Manian A, Randow C, Zehbe R, Fernández-Díaz L, Ziegler A, Fleck C, Schmahl W (2015) Characterization and mechanical properties investigation of the cellulose/gypsum composite. J Compos Mater. https://doi.org/10.1177/0021998315580826
Gong J, Li J, Xu J, Xiang Z, Mo L (2017) Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. https://doi.org/10.1039/C7RA06222B
Hall M, Bansal P, Lee JH, Realff MJ, Bommarius AS (2010) Cellulose crystallinity–a key predictor of the enzymatic hydrolysis rate. FEBS J. https://doi.org/10.1111/j.1742-4658.2010.07585.x
Nasution H, Sitompul S (2017) Preparation and characterization of cellulose microcrystalline (MCC) from fiber of empty fruit bunch palm oil. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/180/1/012007
Almashhadani AQ, Leh CP, Chan S-Y, Lee CY, Goh CF (2022) Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: importance of hydrolysis parameters. Carbohydr Polym 286:119285
Wulandari W, Rochliadi A, Arcana I (2016) Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/107/1/012045
Zhai L, Kim HC, Kim JW, Kim J (2020) Simple centrifugal fractionation to reduce the size distribution of cellulose nanofibers. Sci Rep. https://doi.org/10.1038/s41598-020-68642-7
Herrera MA, Mathew AP, Oksman K (2012) Characterization of cellulose nanowhiskers: a comparison of two industrial bio-residues. IOP Conf Ser: Mater Sci Eng. https://doi.org/10.1088/1757-899X/31/1/012006
Handoko F, Yusuf Y (2021) Synthesis and physicochemical properties of poly(vinyl) alcohol nanocomposites reinforced with nanocrystalline cellulose from tea (Camellia sinensis) waste. Materials. https://doi.org/10.3390/ma14237154
Ng T, Ching YC, Awanis N, Ishenny N, Rahman M (2014) Effect of bleaching condition on thermal properties and UV transmittance of PVA/cellulose biocomposites. Mater Res Innovations. https://doi.org/10.1179/1432891714Z.000000000986
Jakubek ZJ, Chen M, Couillard M, Leng T, Liu L, Zou S, Baxa U, Clogston JD, Hamad WY, Johnston LJ (2018) Characterization challenges for a cellulose nanocrystal reference material: dispersion and particle size distributions. J Nanopart Res. https://doi.org/10.1007/s11051-018-4194-6
El-Sakhawy M, Hassan ML (2007) Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2006.04.009
Alhaji Mohammed M, Basirun WJ, Abd Rahman NM, Shalauddin M, Salleh NM (2022) The effect of acid hydrolysis parameters on the properties of nanocellulose extracted from almond shells. J Nat Fibers 19:14102–14114
Ismail F, Othman NEA, Wahab NA, Aziz AA (2022) The effect of chemical and high pressure homogenization treatment conditions on the morphology of nanocellulose. J Adv Res Appl Mech 93:1–7
Sriruangrungkamol A, Wongjaiyen T, Brostow W, Chonkaew W (2020) Preparation of surface-modified nanocellulose from sugarcane bagasse by concurrent oxalic acid-catalyzed reactions. Chiang Mai Univ J Nat Sci 19:1042–1065
Bhardwaj S, Singh S, Meda RS, Jain S, Maji PK (2023) Structural and morphological exploration of cellulose nanocrystals extracted from lignocellulosic waste biomass of Brassica nigra (mustard straw). Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-023-03970-y
Tarchoun AF, Trache D, Klapötke TM (2019) Microcrystalline cellulose from Posidonia oceanica brown algae: extraction and characterization. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.07.176
Kian L, Saba N, Jawaid M, Fouad H (2020) Characterization of microcrystalline cellulose extracted from olive fiber. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.04.015
Debnath B, Duarah P, Haldar D, Purkait MK (2022) Improving the properties of corn starch films for application as packaging material via reinforcement with microcrystalline cellulose synthesized from elephant grass. Food Packaging Shelf Life. https://doi.org/10.1016/j.fpsl.2022.100937
Zhao T, Chen Z, Lin X, Ren Z, Li B, Zhang Y (2018) Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2017.12.024
Tan WY, Gopinath SC, Anbu P, Velusamy P, Gunny N, Anas A, Chen Y, Subramaniam S (2023) Generation of microcrystalline cellulose from cotton waste and its properties. BioResources. https://doi.org/10.15376/biores.18.3.4884-4896
Adawiyah R, Suryanti V (2022) Preparation and characterization of microcrystalline cellulose from lembang (Typha angustifolia L.). J Phys: Conf Ser. https://doi.org/10.1088/1742-6596/2190/1/012007
Tharazi I, Sulong AB, Muhamad N, Haron CHC, Tholibon D, Ismail NF, Radzi MKFM, Razak Z (2017) Optimization of hot press parameters on tensile strength for unidirectional long kenaf fiber reinforced polylactic-acid composite. Procedia Eng. https://doi.org/10.1016/j.proeng.2017.04.150
Van Hai L, Son HN, Seo YB (2015) Physical and bio-composite properties of nanocrystalline cellulose from wood, cotton linters, cattail, and red algae. Cellulose. https://doi.org/10.1007/s10570-015-0633-z
Faisal M, Žmirić M, Kim NQN, Bruun S, Mariniello L, Famiglietti M, Bordallo HN, Kirkensgaard JJK, Jørgensen B, Ulvskov P (2023) A comparison of cellulose nanocrystals and nanofibers as reinforcements to amylose-based Composite Bioplastics. Coatings 13:1573
Luddee M, Pivsa-Art S, Sirisansaneeyakul S, Pechyen C (2014) Particle size of ground bacterial cellulose affecting mechanical, thermal, and moisture barrier properties of PLA/BC biocomposites. Energy Procedia 56:211–218
Lu T, Liu S, Jiang M, Xu X, Wang Y, Wang Z, Gou J, Hui D, Zhou Z (2014) Effects of modifications of bamboo cellulose fibers on the improved mechanical properties of cellulose reinforced poly(lactic acid) composites. Compos Part B: Eng. https://doi.org/10.1016/j.compositesb.2014.02.030
Huang S, Fu Q, Yan L, Kasal B (2021) Characterization of interfacial properties between fibre and polymer matrix in composite materials: a critical review. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2021.05.076
Rose Joseph M, Nizam P, Gopakumar S, JM H, Vishnu R, Kalarikkal N, Thomas S, Vidyasagaran K, Anoop E (2021) Development and characterization of cellulose nanofibre reinforced Acacia nilotica gum nanocomposite. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2020.113180
Paul UC, Fragouli D, Bayer IS, Zych A, Athanassiou A (2021) Effect of green plasticizer on the performance of microcrystalline cellulose/polylactic acid biocomposites. ACS Appl Polym Mater. https://doi.org/10.1021/acsapm.1c00281
Li Y, Tatum WK, Onorato JW, Zhang Y, Luscombe CK (2018) Low elastic modulus and high charge mobility of low-crystallinity indacenodithiophene-based semiconducting polymers for potential applications in stretchable electronics. Macromolecules. https://doi.org/10.1021/acs.macromol.8b00898
Grząbka-Zasadzińska A, Skrzypczak A, Borysiak S (2019) The influence of the cation type of ionic liquid on the production of nanocrystalline cellulose and mechanical properties of chitosan-based biocomposites. Cellulose. https://doi.org/10.1007/s10570-019-02412-1
Gitari B, Chang BP, Misra M, Navabi A, Mohanty AK (2019) A comparative study on the mechanical, thermal, and water barrier properties of PLA nanocomposite films prepared with bacterial nanocellulose and cellulose nanofibrils. BioResources 14:1867–1889
Sultana T, Sultana S, Nur HP, Khan MW (2020) Studies on mechanical, thermal and morphological properties of betel nut husk nano cellulose reinforced biodegradable polymer composites. J Compos Sci. https://doi.org/10.3390/jcs4030083
Noorbakhsh-Soltani SM, Zerafat MM, Sabbaghi S (2018) A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2018.02.012
Haafiz MM, Hassan A, Zakaria Z, Inuwa IM, Islam MS, Jawaid M (2013) Properties of polylactic acid composites reinforced with oil palm biomass microcrystalline cellulose. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2013.05.069
Jin K, Tang Y, Zhu X, Zhou Y (2020) Polylactic acid based biocomposite films reinforced with silanized nanocrystalline cellulose. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.06.201
Huang L, Zhang X, Xu M, Chen J, Shi Y, Huang C, Wang S, An S, Li C (2018) Preparation and mechanical properties of modified nanocellulose/PLA composites from cassava residue. AIP Adv. https://doi.org/10.1063/1.5023278
Zhang X, Chen L, Mulholland T, Osswald TA (2019) Characterization of mechanical properties and fracture mode of PLA and copper/PLA composite part manufactured by fused deposition modeling. Discover Appl Sci. https://doi.org/10.1007/s42452-019-0639-5
Abral H, Putra GJ, Asrofi M, Park J-W, Kim H-J (2018) Effect of vibration duration of high ultrasound applied to bio-composite while gelatinized on its properties. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2017.08.019
Jabbar A, Militký J, Ali A, Javed MU (2017) Mechanical behavior of nanocellulose coated jute/green epoxy composites. IOP Publishing 254:042015
Pavan MV, Balamurugan K, Balamurugan P (2020) Compressive test fractured surface analysis on PLA-Cu composite filament printed at different FDM conditions. IOP Publishing 988:012019
Petre M, Zarnea G, Adrian P, Gheorghiu E (1999) Biodegradation and bioconversion of cellulose wastes using bacterial and fungal cells immobilized in radiopolymerized hydrogels. Resour, Conserv Recycl. https://doi.org/10.1016/S0921-3449(99)00028-2
Zhijiang C, Ping X, Shiqi H, Cong Z (2019) Soy protein nanoparticles modified bacterial cellulose electrospun nanofiber membrane scaffold by ultrasound-induced self-assembly technique: characterization and cytocompatibility. Cellulose. https://doi.org/10.1007/s10570-019-02513-x
Santmartí A, Lee K (2018) Crystallinity and thermal stability of nanocellulose. CRC, Boca Raton
Chaudhary V, Bajpai PK, Maheshwari S (2018) Effect of moisture absorption on the mechanical performance of natural fiber reinforced woven hybrid bio-composites. J Nat Fibers. https://doi.org/10.1080/15440478.2018.1469451
Mohammed M, Rahman R, Mohammed AM, Adam T, Betar BO, Osman AF, Dahham OS (2022) Surface treatment to improve water repellence and compatibility of natural fiber with polymer matrix: recent advancement. Polym Test. https://doi.org/10.1016/j.polymertesting.2022.107707
Alomayri T, Assaedi H, Shaikh FUA, Low IM (2014) Effect of water absorption on the mechanical properties of cotton fabric-reinforced geopolymer composites. J Asian Ceam Soc. https://doi.org/10.1016/j.jascer.2014.05.005
Muñoz E, García-Manrique JA (2015) Water absorption behaviour and its effect on the mechanical properties of flax fibre reinforced bioepoxy composites. Int J Polym Sci. 2015:390275
Anjali R, Anandha Kumar S, Gangolu J, Abiraami R (2024) Experimental study on self-healing of micro-cracks in concrete with combination of environmentally friendly bacteria. Sustain Struct Build. https://doi.org/10.1007/978-3-031-46688-5_7
Yaacob ND, Ismail H, Ting SS (2016) Soil burial of polylactic acid/paddy straw powder biocomposite. BioResources. https://doi.org/10.15376/biores.11.1.1255-1269
Kim H-S, Kim H-J, Lee J-W, Choi I-G (2006) Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym Degrad Stab. https://doi.org/10.1016/j.polymdegradstab.2005.07.002
Esmizadeh E, Tzoganakis C, Mekonnen TH (2020) Degradation behavior of polypropylene during reprocessing and its biocomposites: thermal and oxidative degradation kinetics. Polymers. https://doi.org/10.3390/polym12081627
Ye G, Li Z, Chen B, Bai X, Chen X, Hu Y (2022) Performance of polylactic acid/polycaprolactone/microcrystalline cellulose biocomposites with different filler contents and maleic anhydride compatibilization. Polym Compos 43:5179–5188
Nasution H, Olaiya NG, Haafiz MM, Abdullah C, Bakar SA, Olaiya FG, Mohamed A, HPS AK (2021) The role of amphiphilic chitosan in hybrid nanocellulose–reinforced polylactic acid biocomposite. Polym Adv Technol 32:3446–3457
Lee S-H, Wang S (2006) Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos Part A: Appl Sci Manufac. https://doi.org/10.1016/j.compositesa.2005.04.015
Abbas L, Nazir F, Gulzar A, Maryam L, Tayyeb A, Iqbal M (2022) Hydroxyapatite and Whitlockite Incorporated Cellulose Reinforced Poly-Caprolactone (PCL): biomimetic nanocomposites for bone tissue engineering applications. J Polym Environ. https://doi.org/10.1007/s10924-022-02717-6
Ruseckaite RA, Jiménez A (2003) Thermal degradation of mixtures of polycaprolactone with cellulose derivatives. Polym Degrad Stab. https://doi.org/10.1016/S0141-3910(03)00106-X
Khalid M, Ratnam CT, Luqman CA, Salmiaton A, Choong TSY, Jalaludin H (2009) Thermal and dynamic mechanical behavior of cellulose- and oil palm empty fruit bunch (OPEFB)-filled polypropylene biocomposites. Polym-Plast Technol Eng. https://doi.org/10.1080/03602550903282986
Gond R, Gupta M, Jawaid M (2021) Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym Compos 42:5400–5412
Ravi babu V, Pugazhenthi G, Katiyar V (2015) Effect of graphene content on the properties of poly(lactic acid) nanocomposites. RSC Adv. https://doi.org/10.1039/C4RA15669B
Onbattuvelli VP, Enneti RK, Simonsen J, Kate KH, Balla VK, Atre SV (2020) Structure and thermal stability of cellulose nanocrystal/polysulfone nanocomposites. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2019.100797
Acknowledgements
The authors wish to thank the Swinburne University of Technology Sarawak campus for providing the instrumentation and equipment support.
Funding
This work was supported by Swinburne Sarawak Strategic Research Grant (SUTS/SoR/RMC/SRG-FECS/2021).
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
W.S. contributed to conceptualization, data curation, formal analysis, investigation, methodology, visualization, and original draft preparation, while Y.L. and P.P. were involved in conceptualization, supervision, validation, and draft review and editing of the writing. K.H. was responsible for funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yong, W.S., Yeu, Y.L., Chung, P.P. et al. Extraction and Characterization of Microcrystalline Cellulose (MCC) from Durian Rind for Biocomposite Application. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03401-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03401-7