Abstract
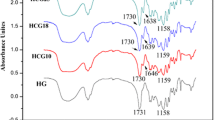
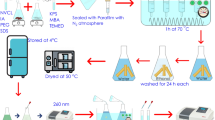
The main objective of the present study was to develop κ-Carragenan (κ-CG) hydrogels by ionic crosslinking, using KCl and NaCl as crosslinkers, as medium controlled releasing of four new cleavable surfactants with antimicrobial activities (C10-MET; C14-MET; C10-BEN and C14-BEN) used as model drugs. The effect of the κ-CG amount on swelling behavior, morphological properties and load/release capacity of surfactants from hydrogels were investigated. The most efficient swelling result was observed for the sample containing a low ratio κ-CG/crosslinker when the swelling medium was distilled water. The hydrogels showed porous structure and elastic solid behavior in the analyzed temperature range (20–200 °C); and were able to load the four drugs by the classic entrapment method. The release results were closely related to the swelling values of the hydrogels and the solubility of the drug in the release medium. It was found that the drug release increased with time, reaching its maximum after 24 h, and C10-MET exhibited the highest percent release. The results of the present work indicate that the κ-CG hydrogels are good support materials for controlled release, with excellent swelling capacity (> 200%), which is a very important property for applications in agriculture.








Similar content being viewed by others
References
Anastas P, Warner J (1998) Principles of green chemistry; Green chemistry: theory and practice. Oxford
Kharissova O, Dias H, Kharisov B, Pérez B, Pérez V (2013) The greener synthesis of nanoparticles. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2013.01.003
Parveen K, Banse V, Ledwani L (2016) Green synthesis of nanoparticles: their advantages and disadvantages. AIP Conf Proc. https://doi.org/10.1063/1.4945168
Kobayashi S (2017) Green polymer chemistry: new methods of polymer synthesis using renewable starting materials. Struct Chem. https://doi.org/10.1007/s11224-016-0861-3
Kharissova O, Kharisov B, Oliva González C, Méndez Y, López I (2019) Greener synthesis of chemical compounds and materials. R Soc Open Sci. https://doi.org/10.1098/rsos.191378
Gowariker V, Viswanathan N, Shreedhar J (2005) Polymer Science. New Age International, New Delhi
Doppalapudi S, Katiyar S, Domb A, Khan W (2014). Adv Polym Med. https://doi.org/10.1007/978-3-319-12478-0
Albuquerque P, Coelho L, Teixeira J, Carneiro-da-Cunha M (2016) Review approaches in biotechnological applications of natural polymers. AIMS Mol Sci. https://doi.org/10.3934/molsci.2016.3.386
Kaushik K, Sharma R, Agarwal S (2016) Natural Polymers and their applications. Int J Pharm Sci Rev Res 37(2):30–36
Ali A, Ahmed S (2018) Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.8b01052
Kouhi M, Prabhakaran M, Ramakrishn S (2020) Edible polymers: an insight into its application in food, biomedicine and cosmetics. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2020.05.025
Tapdiqov S (2020) A drug-loaded gel based on graft radical co-polymerization of Nvinylpyrrolidone and 4-Vinylpyridine with Chitosan. Cellul Chem Technol. https://doi.org/10.35812/CelluloseChemTechnol.2020.54.44
Garcia V, Gonzalez V, Gugliotta L (2019) N, N-dimethylacrylamide hydrogels for controlled drug delivery: influence of network structure and drug solubility on the load and release mechanisms. Lat Am Appl Res. https://doi.org/10.52292/j.laar.2019.365
George J, Hsu C, Nguyen L, Ye H, Cui Z (2019) Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2019.03.009
Gubaidullin A, Makarova A, Derkach S, Voron’ko N, Kadyirov A, Ziganshina S, Salnikov V, Zueva O, Zuev Y (2022) Modulation of molecular structure and mechanical properties of κ-carrageenan-gelatin hydrogel with multi-walled carbon nanotubes. Polymers. https://doi.org/10.3390/polym14122346
Peppas N, Huang Y, Torres-Lugo M, Ward J, Zhang J (2000) Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. https://doi.org/10.1146/annurev.bioeng.2.1.9
Lee K, Mooney D (2001) Hydrogels for tissue engineering. Chem Rev. https://doi.org/10.1021/cr000108x
Zhao S, Ma D, Zhang L (2006) New semi-interpenetrating network hydrogels: synthesis, characterization and properties. Macromol Biosci 1:2. https://doi.org/10.1002/mabi.200600011
Peppas N, Hilt J, Khademhosseini A, Langer R (2006) Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. https://doi.org/10.1002/adma.200501612
Ahmed E (2015) Hydrogel: Preparation, characterization, and applications: a review. J Adv Res. https://doi.org/10.1016/j.jare.2013.07.006
Fu J (2018) Strong and tough hydrogels crosslinked by multi-functional polymer colloids. J Polym Sci Part B. https://doi.org/10.1002/polb.24728
Bashir S, Hina M, Iqbal J, Rajpar A, Mujtaba M, Alghamdi N, Wageh S, Ramesh K, Ramesh S (2020) Fundamental concepts of hydrogels: synthesis, properties, and their applications. Polymers. https://doi.org/10.3390/polym12112702
Bustamante-Torres M, Romero-Fierro D, Arcentales-Vera B, Palomino K, Magaña H, Bucio E (2021) Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels. https://doi.org/10.3390/gels7040182
Tapdiqov S (2021) The bonding nature of the chemical interaction between trypsin and chitosan based carriers in immobilization process depend on entrapped method: a review. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.05.059
Tapdiqov S (2021) Electrostatic and hydrogen bond immobilization of trypsine onto pH-sensitive N-vinylpyrrolidone and 4-vinylpyridine radical co-grafted chitosan based on hydrogel. Macromol Res. https://doi.org/10.1007/s13233-021-9015-6
Coviello T, Matricardi P, Marianecci C, Alhaique F (2007) Polysaccharide hydrogels for modified release formulations. J Controlled Release. https://doi.org/10.1016/j.jconrel.2007.01.004
Campo V, Kawano D, da Silva Jr BD, Carvalho I (2009) Carrageenans: biological properties, chemical modifications and structural analysis—a review. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2009.01.020
Jiao G, Yu G, Zhang J, Ewart H (2011) Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. https://doi.org/10.3390/md9020196
Necas J, Bartosikova L (2013) Carrageenan: a review. Vet Med. https://doi.org/10.17221/6758-VETMED
Pulat M, Yoltay N (2016) Smart fertilizers: preparation and characterization of gelatin-based hydrogels for controlled release of MAP and AN fertilizers. Agrochimica. https://doi.org/10.12871/00021857201641
Rehman W, Majeed A, Rani P, Saini K, Najar R, Mehra R, Singh A, Bast F (2016) In: Saiqa (Ed) Natural Polymers: Derivatives, Blends and Composites. New Delhi, India
Qureshi D, Nayak S, Maji S, Kim D, Banerjee I, Pal K (2019) Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr Pharm Des. https://doi.org/10.2174/1381612825666190425190754
Pacheco-Quito E, Ruiz-Caro R, Veiga M (2020) Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar Drugs. https://doi.org/10.3390/md18110583
Li L, Ni R, Shao Y, Mao S (2014) Carrageenan and its applications in drug delivery. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2013.12.008
BeMiller J (2019) Carbohydrate chemistry for food scientists. AACC International Press, London
Shavit U, Reiss M, Shaviv A (2003) Wetting mechanism of gel based controlled-released fertilizers. JCR. https://doi.org/10.1016/s0168-3659(02)00455-8
Huppert H, Sparks R (2006) Extreme natural hazards: population growth, globalization and environmental change. Philos Trans R Soc A 1:2. https://doi.org/10.1098/rsta.2006.1803
Wu S, Mickley L, Jacob D, Rind D, Streets D (2008) Effects of 2000–2050 changes in climate and emissions on global tropospheric ozone and the policy-relevant background surface ozone in the United States. J Geophys Res. https://doi.org/10.1029/2007JD009639
Cong Z, Yazhen S, Changwen D, Jianmin Z, Huoyan W, Xiaoqin C (2010) Evaluation of waterborne coating for controlled-release fertilizer using wurster fluidized bed. Ind Eng Chem Res. https://doi.org/10.1021/ie101239m
Pulat M, Akalin G (2013) Preparation and characterization of gelatin hydrogel support for immobilization of Candida Rugosa lipase. Artif Cells Nanomed Biotechnol. https://doi.org/10.3109/10731199.2012.696070
Rozo G, Bohorques L, Santamaría J (2019) Controlled release fertilizer encapsulated by a κ-carrageenan hidrogel. Polímeros. https://doi.org/10.1590/0104-1428.02719
Akalin G, Pulat M (2019) Preparation and characterization of κ-carrageenan hydrogel for controlled release of copper and manganese micronutrients. Polym Bull. https://doi.org/10.1007/s00289-019-02800-4
Rizwan M, Gilani S, Durani A, Naseem S (2021) Materials diversity of hydrogel: synthesis, polymerization process and soil conditioning properties in agricultural field. J Adv Res. https://doi.org/10.1016/j.jare.2021.03.007
Liu Y, Wang J, Chen H, Cheng D (2022) Environmentally friendly hydrogel: a review of classification, preparation and application in agriculture. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.157303
Krasnopeeva E, Panova G, Yakimansky A (2022) Agricultural applications of superabsorbent polymer hydrogels. Int J Mol Sci. https://doi.org/10.3390/ijms232315134
Oladosu Y, Rafii M, Arolu F, Chukwu S, Salisu M, Fagbohun I, Muftaudeen T, Swaray S, Haliru B (2022) Superabsorbent polymer hydrogels for sustainable agriculture: a review. Horticulturae. https://doi.org/10.3390/horticulturae8070605
Gilbert E, Guastavino J, Gutierrez C, Lancelle M, Russell-White K, Murguía M (2021) Synthesis and properties of new cleavable cationic surfactants containing carbonate groups. J Surfact Deterg. https://doi.org/10.1002/jsde.12507
Gilbert E, Guastavino J, Nicollier R, Lancelle M, Russell-White K, Murguía M (2021) Synthesis and properties of cleavable quaternary ammonium compounds. J Oleo Sci. https://doi.org/10.5650/jos.ess20216
Jain E, Kumar A (2009) Designing Supermacroporous cryogels based on polyacrylonitrile and a polyacrylamide-chitosan semi-interpenetrating network. J Biomater Sci Polym Ed. https://doi.org/10.1163/156856209X444321
Croitoru C, Pop M, Bedo T, Cosnita M, Roata I, Hulka I (2000) Physically crosslinked poly (vinyl alcohol)/kappa-carrageenan hydrogels: structure and applications. Polymers. https://doi.org/10.3390/polym12030560
Malana M, Zohra R (2013) The release behavior and kinetic evaluation of tramadol HCl from chemically crosslinked Ter polymeric hydrogels. Daru. https://doi.org/10.1186/2008-2231-21-10
Peppas N, Bunes P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. https://doi.org/10.1016/s0939-6411(00)00090-4
Primo G, GarciaManzano M, Romero M, AlvarezIgarzabal C (2015) Synthesis and characterization of hydrogels from 1-vinylimidazole. Highly resistant co-polymers with synergistic effect. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2015.01.027
Peppas N, Khare A (1993) Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv Drug Deliv Rev. https://doi.org/10.1016/0169-409X(93)90025-Y
Serra L, Doménech J, Peppas N (2006) Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials. https://doi.org/10.1016/j.biomaterials.2006.06.011
Fu Y, Kao W (2010) Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. https://doi.org/10.1517/17425241003602259
Siepmann J, Peppas N (2012) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2012.09.028
Rasool A, Ata S, Islam A, Khan R (2019) Fabrication of novel carrageenan based stimuli responsive injectable hydrogels for controlled release of cephradine. RSC Adv. https://doi.org/10.1039/c9ra02130b
Santo V, Frias A, Carida M, Cancedda R, Gomes M, Mano J, Reis R (2009) Carrageenan-based hydrogels for the controlled delivery of PDGF-BB in bone tissue engineering applications. Biomacromolecules. https://doi.org/10.1021/bm8014973
Braudo E (2004) Mechanism of galactan gelation. Food Hydrocoll. https://doi.org/10.1016/S0268-005X(09)80056-8
Kelco CP (2004) Product catalogue GENU®; Carrageenan Book; www.cpkelco.com
Gulrez S, Al-Assaf S, Phillips G (2011) Progress in molecular and environmental bioengineering—from analysis and modeling to technology applications. InTechOpen
Rhein-Knudsen N, Ale M, Meyer A (2015) Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar Drugs. https://doi.org/10.3390/md13063340
Rioux L, Turgeon S (2015) Seaweed carbohydrates. Elsevier Inc., Amsterdam
Graham S, Marina P, Blencowe A (2019) Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2018.11.053
Bui V, Nguyen B, Nicolai T, Renou F (2019) Mixed iota and kappa carrageenan gels in the presence of both calcium and potassium ions. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115107
Saidin S, Mobarak N (2020) Hydrogel of kappa-carrageenan as adsorbent for methylene blue. J Mater Sci Technol 5(1):1–12
Khare A, Peppas N (1995) Swelling/deswelling of anionic copolymer gels. Biomaterials. https://doi.org/10.1016/0142-9612(95)91130-Q
Lin Y, Liang H, Chung C, Chen M, Sung H (2005) Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials. https://doi.org/10.1016/j.biomaterials.2004.06.011
Shahbazi M, Ettelaie R, Rajabzadeh G (2016) Physico-mechanical analysis data in support of compatibility of chitosan/κ-carrageenan polyelectrolyte films achieved by ascorbic acid, and the thermal degradation theory of κ-carrageenan influencing the properties of its blends. Data Brief. https://doi.org/10.1016/j.dib.2016.09.039
Hermansson A, Eriksson E, Jordansson E (1991) Effects of potassium, sodium and calcium on the microstructure and rheologicai behaviour of kappa-carrageenan gels. Carbohydr Polym. https://doi.org/10.1016/0144-8617(91)90115-S
Chew K (1980) Relation between composition and physical properties of carrageenan gums. Thesis for the degree of Master of Science
Mangione M, Giacomazza D, Bulone D, Martorana V, Cavallaro G, San Biagio P (2005) K+ and Na+ effects on the gelation properties of κ-Carrageenan. Biophys Chem. https://doi.org/10.1016/j.bpc.2004.08.005
Mangione M, Giacomazza D, Bulone D, Martorana V, San Biagio P (2003) Thermoreversible gelation of κ-Carrageenan: relation between conformational transition and aggregation. Biophys Chem. https://doi.org/10.1016/S0301-4622(02)00341-1
Graessley W (1974) Advances in polymer science. Springer, Berlin
Porter R, Johnson J (1966) The entanglement concept in polymer systems. Chem Rev. https://doi.org/10.1021/cr60239a001
Mcleish T (2002) Tube theory of entangled polymer dynamics. Adv Phys. https://doi.org/10.1080/00018730210153216
Puza F, Zheng Y, Han L, Xue L, Cui J (2020) Physical entanglement hydrogels: ultrahigh water content but good toughness and stretchability. Polym Chem. https://doi.org/10.1039/DOPY00294A
Gong J, Katsuyama Y, Kurokawa T, Osada Y (2003) Double-network hydrogels with extremely high mechanical strength. Adv Mater. https://doi.org/10.1002/adma.200304907
Francis S, Kumar M, Varshney L (2004) Radiation synthesis of superabsorbent poly(acrylic acid)–carrageenan hydrogels. Radiat Phys Chem. https://doi.org/10.1016/j.radphyschem.2003.09.004
Bixler H (1994) The carrageenan connection IV. Br Food J. https://doi.org/10.1108/00070709410060763
Sharma S, Dua A, Malik A (2014) Polyaspartic acid based superabsorbent polymers. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2014.07.043
Nourmohammadi J, Roshanfar F, Farokhi M, Haghbin M (2017) Silk fibroin/kappa -carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2017.03.166
Berton S, de Jesus G, Sabino R, Monteiro J, Venter S, Bruschi M, Popat K, Matsushita M, Martins A, Bonafé E (2020) Properties of a commercial κ-carrageenan food ingredient and its durable superabsorbent hydrogels. Carbohydr Res. https://doi.org/10.1016/j.carres.2019.107883
Marcus Y (1988) Ionic radii in aqueous solutions. Chem Rev. https://doi.org/10.1021/cr00090a003
Cao Y, Li S, Fang Y, Nishinari K, Phillips G, Lerbret A, Assifaoui A (2018) Specific binding of trivalent metal ions to λ-carrageenan. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2017.12.095
López-Avila A, Rodríguez-Manzo G, Coffeen U, del Angel R, Pellicer F (2004) Self-injury behaviour induced by intraplantar carrageenan infiltration: a model of tonic nociception. Brain Res Brain Res Protoc. https://doi.org/10.1016/j.brainresprot.2004.01.001
Mancinelli R, Botti A, Bruni F, Ricci M, Soper A (2007) Perturbation of water structure due to monovalent ions in solution. Phys Chem Chem Phys. https://doi.org/10.1039/B701855J
Mancinelli R, Botti A, Bruni F, Ricci M (2007) Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J Phys Chem B. https://doi.org/10.1021/jp075913v
Marcus Y (2009) Effect of ions on the structure of water: structure making and breaking. Chem Rev. https://doi.org/10.1021/cr8003828
Lin C, Metters A (2006) Hydrogels in controlled release formulations: network design and mathematical modelling. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2006.09.004
Funding
Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT), Universidad Nacional del Litoral (UNL) and Agencia Santafesina de Ciencia, Tecnología e Innovación (ASaCTeI).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. All authors will participate in the writing of the manuscript. All authors participated in the development of the experimental part
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia, V.S., Gugliotta, L.M., Gutierrez, C.G. et al. κ-Carrageenan Hydrogels as a Sustainable Alternative for Controlled Release of New Biodegradable Molecules with Antimicrobial Activities. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03189-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03189-6