Abstract
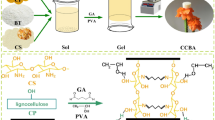
Montmorillonite-based porous adsorbent prepared by gel casting method was used to adsorb Cr(III)-organic complexes in tanning wastewater,together with the initial concentration of 10 mg L−1. The as-porous adsorbent exhibited an excellent performance of separation. Meanwhile, its structural and morphology were characterized by TG, BET, XRD, SEM, EDS and XPS, showing that the porous adsorbent was complete hollow ball structure. And the adsorption results showed that the adsorption amount of porous adsorbent rapidly declined from 9.58 to 5.28 mg L−1, and only Cr(III) was adsorbed, when the molar ratio of Cr(III) and citrate was more than 1:5. Increased of pH was beneficial to adsorb Cr(III)-citrate in range from 2.46 to 7.12. Furthermore, the best removal efficiency of Cr(III)-organic complex was up to 97% and still above 84% after five cycles with porous adsorbent. Finally, the mechanism on adsorbing Cr(III) with porous adsorbent is also provided. Therefore, a kind of porous adsorbent which was easily separated, was prepared to deeply treat tanning wastewater containing Cr(III)-organic complex, and solve the problem of difficult recycle.








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lofrano G, Meric S, Zengin GE, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 461–462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
Covington AD (1997) Modern tanning chemistry. Chem Soc Rev 26:111. https://pubs.rsc.org/en/content/articlehtml/1997/cs/cs9972600111
He X, Wu C, Qian Y, Li Y, Zhang L, Ding F, Chen H, Shen J (2019) Highly sensitive and selective light-up fluorescent probe for monitoring gallium and chromium ions in vitro and in vivo. Analyst 144:3807–3816. https://doi.org/10.1039/c9an00625g
He X, Chen H, Xu C, Fan J, Xu W, Li Y, Deng H, Shen J (2020) Ratiometric and colorimetric fluorescent probe for hypochlorite monitor and application for bioimaging in living cells, bacteria and zebrafish. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.122029
He X, Deng Z, Xu W, Li Y, Xu C, Chen H, Shen J (2020) A novel dual-response chemosensor for bioimaging of exogenous/endogenous hypochlorite and hydrazine in living cells, pseudomonas aeruginosa and zebrafish. Sens Actuators B. https://doi.org/10.1016/j.snb.2020.128450
He X, Xu C, Xiong W, Qian Y, Fan J, Ding F, Deng H, Chen H, Shen J (2019) The ICT-based fluorescence and colorimetric dual sensing of endogenous hypochlorite in living cells, bacteria, and zebrafish. Analyst 145:29–33. https://doi.org/10.1039/c9an02226k
Hokkanen S, Bhatnagar A, Repo E, Lou S, Sillanpää M (2016) Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem Eng J 283:445–452. https://doi.org/10.1016/j.cej.2015.07.035
Meunier N, Drogui P, Montane C, Hausler R, Mercier G, Blais JF (2006) Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J Hazard Mater 137:581–590. https://doi.org/10.1016/j.jhazmat.2006.02.050
Yang Y, Wang G, Deng Q, Ng DH, Zhao H (2014) Microwave-assisted fabrication of nanoparticulate TiO2 microspheres for synergistic photocatalytic removal of Cr(VI) and methyl orange. ACS Appl Mater Inter 6:3008–3015. https://doi.org/10.1021/am405607h
Religa P, Kowalik A, Gierycz P (2011) Application of nanofiltration for chromium concentration in the tannery wastewater. J Hazard Mater 186:288–292. https://doi.org/10.1016/j.jhazmat.2010.10.112
Wang D, Zhang G, Dai Z, Zhou L, Bian P, Zheng K, Wu Z, Cai D (2018) Sandwich-like nanosystem for simultaneous removal of Cr(VI) and Cd(II) from water and soil. ACS Appl Mater Inter 10:18316–18326. https://doi.org/10.1021/acsami.8b03379
Elabbas S, Mandi L, Berrekhis F, Pons MN, Leclerc JP, Ouazzani N (2016) Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J Environ Manage 166:589–595. https://doi.org/10.1016/j.jenvman.2015.11.012
Liu J, Huang K, Xie K, Yang Y, Liu H (2016) An ecological new approach for treating Cr(VI)-containing industrial wastewater: photochemical reduction. Water Res 93:187–194. https://doi.org/10.1016/j.watres.2016.02.025
Ye Y, Jiang Z, Xu Z, Zhang X, Wang D, Lv L, Pan B (2017) Efficient removal of Cr(III)-organic complexes from water using UV/Fe(III) system: negligible Cr(VI) accumulation and mechanism. Water Res 126:172–178. https://doi.org/10.1016/j.watres.2017.09.021
Wang D, Ye Y, Liu H, Ma H, Zhang W (2018) Effect of alkaline precipitation on Cr species of Cr(III)-bearing complexes typically used in the tannery industry. Chemosphere 193:42–49. https://doi.org/10.1016/j.chemosphere.2017.11.006
Qiu J, Dong S, Wang H, Cheng X, Du Z (2015) Adsorption performance of low-cost gelatin–montmorillonite nanocomposite for Cr(III) ions. RSC Adv 5:58284–58291. https://pubs.rsc.org/en/content/articlepdf/2015/ra/c5ra08781c
Ijagbemi CO, Baek MH, Kim DS (2009) Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J Hazard Mater 166:538–546. https://doi.org/10.1016/j.jhazmat.2008.11.085
Dong Z, Zhang F, Wang D, Liu X, Jin J (2015) Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J Solid State Chem 224:88–93. https://doi.org/10.1016/j.jssc.2014.06.030
Abou-El-Sherbini KS, Hassanien MM (2010) Study of organically-modified montmorillonite clay for the removal of copper(II). J Hazard Mater 184:654–661. https://doi.org/10.1016/j.jhazmat.2010.08.088
Di Natale F, Erto A, Lancia A, Musmarra D (2015) Equilibrium and dynamic study on hexavalent chromium adsorption onto activated carbon. J Hazard Mater 281:47–55. https://doi.org/10.1016/j.jhazmat.2014.07.072
Gong K, Hu Q, Yao L, Li M, Sun D, Shao Q, Qiu B, Guo Z (2018) Ultrasonic pretreated sludge derived stable magnetic active carbon for Cr(VI) removal from wastewater. ACS Sustainable Chem Eng 6:7283–7291. https://doi.org/10.1021/acssuschemeng.7b04421
Yu P, Wang H-Q, Bao R-Y, Liu Z, Yang W, Xie B-H, Yang M-B (2017) Self-assembled sponge-like chitosan/reduced graphene oxide/montmorillonite composite hydrogels without cross-linking of chitosan for effective Cr(VI) sorption. ACS Sustainable Chem Eng 5:1557–1566. https://doi.org/10.1021/acssuschemeng.6b02254
Cheng W, Ding C, Nie X, Duan T, Ding R (2017) Fabrication of 3D macroscopic graphene oxide composites supported by montmorillonite for efficient U(VI) wastewater purification. ACS Sustainable Chem Eng 5:5503–5511. https://doi.org/10.1021/acssuschemeng.7b00841
Zhu J, Cozzolino V, Pigna M, Huang Q, Caporale AG, Violante A (2011) Sorption of Cu, Pb and Cr on Na-montmorillonite: competition and effect of major elements. Chemosphere 84:484–489. https://doi.org/10.1016/j.chemosphere.2011.03.020
Jin J, Xiao T, Tan Y, Zheng J, Liu R, Qian G, Wei H, Zhang J (2018) Effects of TiO2 pillared montmorillonite nanocomposites on the properties of asphalt with exhaust catalytic capacity. J Clean Prod 205:339–349. https://doi.org/10.1016/j.jclepro.2018.08.251
Dou X, Mohan D, Pittman CU, Yang S (2012) Remediating fluoride from water using hydrous zirconium oxide. Chem Eng J 198–199:236–245. https://doi.org/10.1016/j.cej.2012.05.084
Reddy CV, Babu B, Reddy IN, Shim J (2018) Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram Int 44:6940–6948. https://doi.org/10.1016/j.ceramint.2018.01.123
Ma ET, Yang D, Sun S, Xu Y, Kim J EJ (2019) Zirconium dioxide loaded montmorillonite composites as high-efficient adsorbents for the removal of Cr3+ ions from tanning wastewater. J Solid State Chem 277:502–509. https://doi.org/10.1016/j.jssc.2019.07.002
Zhang F-Z, Kato T, Fuji M, Takahashi M (2006) Gelcasting fabrication of porous ceramics using a continuous process. J Eur Ceram Soc 26:667–671. https://doi.org/10.1016/j.jeurceramsoc.2005.07.021
Li ET, Yang Y, Qian S, Liu J, Xing L J (2018) Nano-montmorillonite‐based porous material prepared by gel casting: structure and adsorption properties. Micro Nano Lett 13:332–334. https://doi.org/10.1049/mnl.2017.0531
Alsbaiee A, Smith BJ, Xiao L, Ling Y, Helbling DE, Dichtel WR (2016) Rapid removal of organic micropollutants from water by a porous beta-cyclodextrin polymer. Nature 529:190–194. https://doi.org/10.1038/nature16185
Gustafsson JP, Persson I, Oromieh AG, van Schaik JW, Sjostedt C, Kleja DB (2014) Chromium(III) complexation to natural organic matter: mechanisms and modeling. Environ Sci Technol 48:1753–1761. https://doi.org/10.1021/es404557e
Chen N, Pan B (2021) Tributylhexadecylphosphonium modification strategy to construct gold nanoprobes for the detection of aqueous Cr(III)-organic complexes. Anal Chem 93:1811–1817. https://doi.org/10.1021/acs.analchem.0c04688
Zhu L, Wang L, Xu Y (2017) Chitosan and surfactant co-modified montmorillonite: a multifunctional adsorbent for contaminant removal. Appl Clay Sci 146:35–42. https://doi.org/10.1016/j.clay.2017.05.027
Zhang G, He Z, Xu W (2012) A low-cost and high efficient zirconium-modified-Na-attapulgite adsorbent for fluoride removal from aqueous solutions. Chem Eng J 183:315–324. https://doi.org/10.1016/j.cej.2011.12.085
Saha I, Ghosh A, Nandi D, Gupta K, Chatterjee D, Ghosh UC (2015) β-Cyclodextrin modified hydrous zirconium oxide: synthesis, characterization and defluoridation performance from aqueous solution. Chem Eng J 263:220–230. https://doi.org/10.1016/j.cej.2014.11.039
Parashar K, Ballav N, Debnath S, Pillay K, Maity A (2017) Hydrous ZrO2 decorated polyaniline nanofibres: synthesis, characterization and application as an efficient adsorbent for water defluoridation. J Colloid Interface Sci 508:342–358. https://doi.org/10.1016/j.jcis.2017.08.044
Lutfullah, Rashid M, Haseen U, Rahman N (2014) An advanced Cr(III) selective nano-composite cation exchanger: synthesis, characterization and sorption characteristics. J Ind Eng Chem 20:809–817. https://doi.org/10.1016/j.jiec.2013.06.010
Dhanpat R, Bruce MS, Moore DA (1987) Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorg Chem 26:345–349. https://doi.org/10.1021/ic00250a002
Pan X, Cheng S, Zhang C, Jiao Y, Lin X, Dong W, Qi X (2021) Mussel-inspired magnetic pullulan hydrogels for enhancing catalytic degradation of antibiotics from biomedical wastewater. Chem Eng J 409. https://doi.org/10.1016/j.cej.2020.128203
Qi X, Zeng Q, Tong X, Su T, Xie L, Yuan K, Xu J, Shen J (2021) Polydopamine/montmorillonite-embedded pullulan hydrogels as efficient adsorbents for removing crystal violet. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123359
Su T, Wu L, Pan X, Zhang C, Shi M, Gao R, Qi X, Dong W (2019) Pullulan-derived nanocomposite hydrogels for wastewater remediation: synthesis and characterization. J Colloid Interface Sci 542:253–262. https://doi.org/10.1016/j.jcis.2019.02.025
Qi X, Liu R, Chen M, Li Z, Qin T, Qian Y, Zhao S, Liu M, Zeng Q, Shen J (2019) Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr Polym 209:101–110. https://doi.org/10.1016/j.carbpol.2019.01.015
Wu P, Zhang Q, Dai Y, Zhu N, Dang Z, Li P, Wu J, Wang X (2011) Adsorption of Cu(II), Cd(II) and Cr(III) ions from aqueous solutions on humic acid modified Ca-montmorillonite. Geoderma 164:215–219. https://doi.org/10.1016/j.geoderma.2011.06.012
Gomez-Gonzalez SE, Carbajal-Arizaga GG, Manriquez-Gonzalez R, De la Cruz-Hernandez W, Gomez-Salazar S (2014) Trivalent chromium removal from aqueous solutions by a sol–gel synthesized silica adsorbent functionalized with sulphonic acid groups. Mater Res Bull 59:394–404. https://doi.org/10.1016/j.materresbull.2014.07.035
Lesaoana M, Pakade VE, Chimuka L (2019) Crosslinker-less surface-imprinted Macadamia derived activated carbons for trace Cr(III) removal from aqueous solution. Environ Inno. https://doi.org/10.1016/j.eti.2019.100336
Fonseca-Correa R, Giraldo L, Moreno-Piraján JC (2013) Trivalent chromium removal from aqueous solution with physically and chemically modified corncob waste. J Anal Appl Pyrolysis 101:132–141. https://doi.org/10.1016/j.jaap.2013.01.019
Zhang F, Lan J, Zhao Z, Yang Y, Tan R, Song W (2012) Removal of heavy metal ions from aqueous solution using Fe3O4-SiO2-poly(1,2-diaminobenzene) core-shell sub-micron particles. J Colloid Interface Sci 387:205–212. https://doi.org/10.1016/j.jcis.2012.07.066
Acknowledgements
The research was supported by Liaoning Province (Jinzhou) Fur Green Manufacturing Industry Technology Innovation Strategic Alliance [201854], Research on resource recycling technology of tanned chromium-containing waste dander [2018020190-301], The “Seedling” Project named “Research on Graphene-based 3D Material Construction and Key Technologies for Industrialization Application” for Youth Science and Technology Talents of Education Department of Liaoning Province (LQ2020011), Study on the deep removal of heavy metal chromium from industrial wastewater in the upper reaches of Daling River estuary [BDHYYJY2020013] (This study were supported by the Open Fund of Institute of Ocean Research, Bohai University), National Natural Science Foundation of China [21878024], Innovation Team Project of Liaoning Province [LT2015001], and Innovation Team Project of Liaoning Higher University [2018479-14].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service or company. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Hao, X., Tao E, Yang, S. et al. A New Montmorillonite-Based Porous Composites: Effectively Removal of Cr(III)-Organic Complexes in Tannery Wastewater. J Polym Environ 30, 308–318 (2022). https://doi.org/10.1007/s10924-021-02193-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02193-4