Abstract
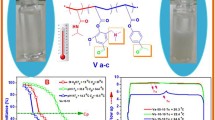
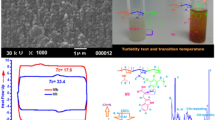
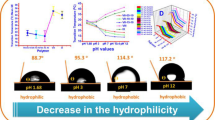
To optimize the phase separation temperature of poly (N-isopropylacrylamide) two monomers have been used to fabricate a series of three functional and thermo-pH terpolymers. N-(2-(dimethylamino)ethyl)acrylamide (DMAEAm) acts as a hydrophilic and pH-responsive monomer and 4-formyl-2-methoxyphenylacrylate or vanillin acrylate (VA) as hydrophobic and functional monomer both monomers have been synthesized in a one-step reaction. They were investigated by 1H, 13C NMR, and FTIR and demonstrated good agreement with their chemical structures. Dual responsive functional terpolymers have been fabricated in different molar ratios of (DMAEAm) (10, 15, and 20 mol%) with 10 mol% of VA; they have been investigated by chemical methods such as 1H, and FTIR as well. The physical characterization has also been achieved using GPC for molecular weight and molecular weight distributions, DSC for glass transition temperature. The vital point is studying the phase separation temperature or the lower critical solution temperature of the polymer solution, and the relative influence of DMAEAm and VA on transition temperature Tc of terpolymer solution; it has been recorded by turbidity test using UV–Vis-spectroscopy as the change of transmittance with temperature, on the other hand using micro-DSC of the polymer solution was tested. This optimization in the poly (NIPAAm) characterization encourages the application of the new terpolymer as a biosensor.
Graphic Abstract
















Similar content being viewed by others
References
Elizabeth RG (2020) Reflections on the evolution of smart polymers. Isr J Chem 60:75–85. https://doi.org/10.1002/ijch.201900075
Tanaka M, Nakahata M, Linke P, Philipp L, Stefan K (2020) Stimuli-responsive hydrogels as a model of the dynamic cellular microenvironment. Polym J 52:861–870. https://doi.org/10.1038/s41428-020-0353-6
Abdelaty MSA (2020) The effect hydrophilic/hydrophobic interaction of 2 ((dimethylamino)methyl) 4 formyl 6 methoxyphenyl acrylate and 4 acetylphenyl acrylate monomers on the phase transition temperature of N isopropylacrylamide terpolymers. J Polym Environ 28:2584–2598. https://doi.org/10.1007/s10924-020-01790-z
Abdelaty MSA (2020) the influence of vanillin acrylate derivative on the phase separation temperature of environmental photo-cross-linked N-isopropylacrylamide copolymer and hydrogel thin films. J Polym Environ 28:2599–2615. https://doi.org/10.1007/s10924-020-01793-w
Abdelaty MSA (2020) Influence of vanillin acrylate and 4-acetylphenyl acrylate hydrophobic functional monomers on phase separation of N-isopropylacrylamide environmental terpolymer: fabrication and characterization. Polym Bull 77:2905–2922. https://doi.org/10.1007/s00289-019-02890-0
Huanqing C, Qilong Z, Li Z, Xuemin D (2020) Intelligent polymer-based bioinspired actuators: from monofunction to multifunction. Adv Intell Syst 2000138:1–15. https://doi.org/10.1002/aisy.202000138
Chen J-K, Chang C-J (2014) Fabrication and applications of stimuli-responsive polymer films and patterns on surface. Materials 7:805–875. https://doi.org/10.3390/ma7020805
Mengle K, Xinwen P, Hao C, Peiwen L, Bo P, Kai Z (2020) pH-responsive polymeric nanoparticles with tunable sizes for targeted drug delivery. RSC Adv 10:4860–4868
Naruphorn D, Farzad S, Juliette H, Daniel C (2020) Controlling release kinetics of pH-responsive polymer nanoparticles. Polym Chem 11:1752–1762. https://doi.org/10.1039/C9PY01946D
Nayeleh D, Changhe Z, Sarah SK, Angus PRJ, Georgina KS (2019) pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun 40:e1800917. https://doi.org/10.1002/marc.201800917
Hyuk L, Hongsuk P, Gwang JN, Eun SL (2018) pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr Polym 202:323–333. https://doi.org/10.1016/j.carbpol.2018.08.141
Sijie X, Matthew JW (2020) Temperature-responsive supramolecular hydrogels. J Mater Chem B 1:11. https://doi.org/10.1039/D0TB01814G
Liu Z, Zhang S, He B, Shoujuan W, Fangong K (2020) Temperature-responsive hydroxypropyl methylcellulose-N-isopropylacrylamide aerogels for drug delivery systems. Cellulose. https://doi.org/10.1007/s10570-020-03426-w
Wang Y, Gong J, Hu W (2020) Transparency of temperature-responsive shape-memory gels tuned by a competition between crystallization and glass transition. Chin J Polym Sci. https://doi.org/10.1007/s10118-020-2456-0
Augustinus JJK, Nadia CMZ, Dirk JB, Albert PHJS (2019) Temperature-responsive, multicolor-changing photonic. Polym ACS Appl Mater Interfaces 31:28172–28179. https://doi.org/10.1021/acsami.9b08827
Qiao S, Wang H (2018) Temperature-responsive polymers: synthesis, properties, and biomedical applications. Nano Res 11:5400–5423. https://doi.org/10.1007/s12274-018-2121-x
Tao X, Ting L, Wei-Feng Z, Cheng-Sheng Z (2019) Ionic-strength responsive zwitterionic copolymer hydrogels with tunable swelling and adsorption behaviors. Langmuir 35:1146–1155
Fatma Ç, Papatya K, Grace A, Zeynep AA (2020) Ionic strength-responsive poly(sulfobetaine methacrylate) microgels for fouling removal during ultrafiltration. React Funct Polym 156:104738. https://doi.org/10.1016/j.reactfunctpolym.2020.104738
Aleksey DD, Jesper de Claville C (2020) Mechanical response and equilibrium swelling of thermoresponsive copolymer hydrogels. Polym Int 69:974–984
Wang J, Suzuki R, Ogata K, Nakamura T, Dong A, Weng W (2020) Near-linear responsive and wide-range pressure and stretch sensor based on hierarchical graphene-based structures via solvent-free preparation. Polymers 12:1814. https://doi.org/10.3390/polym12081814
Valentina M, Pierfrancesco C, Marta G, Veronica BT (2017) Light-responsive polymer micro- and nano-capsules. Polymers 9:1–19. https://doi.org/10.3390/polym9010008
Zhou C, Shi Z, Xu F, Ying L, Haoyu T (2020) Preparation and properties of thermo- and pH-responsive polypeptide bearing OEG and aldehyde pendants. Colloid Polym Sci 298:1293–1302. https://doi.org/10.1007/s00396-020-04712-6
Castro-Hernández A, Cortez-Lemus NA (2019) Thermo/pH responsive star and linear copolymers containing a cholic acid-derived monomer, N-isopropylacrylamide and acrylic acid: synthesis and solution properties. Polymers 11:1859. https://doi.org/10.3390/polym11111859
Dirk S (2006) Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev 58:1655–1670
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin (part 2): temperature responsive layer A, functional, temperature and pH layer B. Polym Bull 11:4837–4858. https://doi.org/10.1007/s00289-018-2297-y
Weizhong Y, Wen G, Hui Z, Jie R (2013) Tunable thermo-, pH- and light-responsive copolymer micelles. Polym Chem 4:3934–3937. https://doi.org/10.1039/C3PY00478C
Shan S, Qianman W, Tao W, Shuping R, Yu G, Na W (2014) Thermo-, pH-, and light-responsive poly(N-isopropylacrylamide-co-methacrylic acid)–Au hybrid microgels prepared by the in situ reduction method based on Au-thiol chemistry. J Phys Chem B 118:7177–7186. https://doi.org/10.1021/jp5027477
Tao X, Ting L, Wei-Feng Z, Chang-Sheng Z (2018) Ionic strength- and thermo-responsive polyethersulfone composite membranes with enhanced antifouling properties. New J Chem 42:5323–5333. https://doi.org/10.1039/C8NJ00039E
St K, Ilja V, Gerhard F-P, Gregor T (2009) pH and ionic strength responsive polyelectrolyte block copolymer micelles prepared by ring opening metathesis polymerization. J Polym Sci A 47:1178–1191. https://doi.org/10.1002/pola.23229
Fujian H, Wei-Ching L, Yang Sung S, Rachel N, Chun-Hua L, Itamar W (2016) Light-responsive and pH-responsive DNA microcapsules for controlled release of loads. J Am Chem Soc 138:8936–8945. https://doi.org/10.1021/jacs.6b04773
Ying L, Hongmei C, Dian L, Wenxi W, Ye L, Shaobing Z (2015) pH-responsive shape memory poly(ethylene glycol)–poly(ε-caprolactone)-based polyurethane/cellulose nanocrystals nanocomposite. ACS Appl Mater Interfaces 23:12988–12999
Shibayama M, Tanaka T (1993) Volume phase transition and related phenomena of polymer gels. Adv Polym Sci 109:1–62. https://doi.org/10.1007/3-540-56791-7-1
Shengyi D, Jan H, Jiayin Y, Christoph AS (2016) Lower critical solution temperature (LCST) phase behavior of an ionic liquid and its control by supramolecular host-guest interactions. Chem Commun 52:7970–7973
Fei W, Patrick A, Lorenz R, Haodong Z, Michael S, Britta N (2019) Progress report on phase separation in polymer solutions. Adv Mater 31:1806733. https://doi.org/10.1002/adma.201806733
Jan S, Seema A (2013) Polymers with upper critical solution temperature in aqueous solution: unexpected properties from known building blocks. ACS Macro Lett 7:597–600. https://doi.org/10.1021/mz400227y
Heskins M, Guillet JE (1968) Solution properties of poly(N-isopropylacrylamide). J Macromol Sci 2:1441–1455. https://doi.org/10.1080/10601326808051910
Xia J, Dubin PL (1994) Protein–polyelectrolyte complexes. In: Dubin P, Bock J, Davis R, Schulz DN, Thies C (eds) Macromolecular complexes in chemistry and biology. Springer, Berlin
Kawamura A, Miyata T (2014) pH-responsive polymer. Encyclopedia of polymeric nanomaterials. Springer, Berlin
Yang L, Zhaohui W, Qi W, Min L, Gang H, Baran DS, Jinming G (2016) Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems. Polym Chem 7:5949–5956. https://doi.org/10.1039/C6PY01104G
Zefeng S, Ke W, Chengqiang G, Shuang W, Wangqing Z (2016) A new thermo-, pH-, and CO2-responsive homopolymer of poly[N-[2-(diethylamino)ethyl]acrylamide]: is the diethylamino group underestimated? Macromolecules 49:162–171
Abdelaty MSA (2018) Environmental functional photo-cross-linked hydrogel bilayer thin films from vanillin. J Polym Environ 26:2243–2256. https://doi.org/10.1007/s10924-017-1126-y
Abdelaty MSA (2019) Layer by layer photo-cross-linked environmental functional hydrogel thin films based on vanillin: part 3. J Polym Environ 27:1212–1225. https://doi.org/10.1007/s10924-019-01421-2
Benrebouh A, Avoce D, Zhu XX (2001) Thermo- and pH-sensitive polymers containing cholic acid derivatives. Polymer 42:4031–4038. https://doi.org/10.1016/S0032-3861(00)00837-5
Kocak G, Tuncer C, Bütün V (2017) pH-Responsive polymers. Polym Chem 8:144–176. https://doi.org/10.1039/C6PY01872F
Ramkissoon-Ganorkar C, Baudys M, Wan Kim S (2000) Effect of ionic strength on the loading efficiency” of the model polypeptide/protein drugs in pH-/temperature-sensitive polymers. J Biomat Sci Polym Ed 11:45–54
Ju HK, Kim SY, Kim SJ, Lee YM (2002) pH/temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide. J Appl Polym Sci 11:28–1139. https://doi.org/10.1002/app.10137
Sarwan T, Kumar P, Choonara YE, Pillay V (2020) Hybrid thermo-responsive polymer systems and their biomedical applications. Front Mater 7:73. https://doi.org/10.3389/fmats.2020.00073
Abdelaty MSA (2018) Preparation and characterization of new environmental functional polymers based on vanillin and N-isopropylacrylamide for post polymerization. J Polym Environ 26:636–646. https://doi.org/10.1007/s10924-017-0960-2
Abdelaty MSA, Kuckling D (2018) Poly (N-isopropyl acrylamide-co-vanillin acrylate) dual responsive functional copolymers for grafting biomolecules by Schiff’s base click reaction. Open J Org Polym Mater 8:15–32. https://doi.org/10.4236/ojopm.2018.82002
Patrick V, Holger F (2020) Amino-functional polyethers: versatile, stimuli-responsive polymers. Polym Chem 11:3940–3950. https://doi.org/10.1039/D0PY00466A
Yiwen P, Odilia RS, Jing Yang Q, Peter JR, Andrew BL (2015) pH-, thermo- and electrolyte-responsive polymer gels derived from a well-defined, RAFT-synthesized, poly(2-vinyl-4,4-dimethylazlactone) homopolymer via one-pot post-polymerization modification. Eur Polym J 62:204–213
Camille L, De Marie-Claire P-G, Kam Chiu T, Sébastien L, Daniel T (2015) Aldehyde-functional copolymers based on poly(2-oxazoline) for post-polymerization modification. Eur Polym J 62:322–330. https://doi.org/10.1016/j.eurpolymj.2014.08.026
Matthias C, Thomas J (2011) Photoinduced conjugation of aldehyde functional polymers with olefins via [2 + 2]-cycloaddition. Macromolecules 44(20):7969–7976. https://doi.org/10.1021/ma2017748
Fache M, Darroman E, Besse V, Auvergne R, Sylvain Caillol S, Boutevina B (2014) Vanillin, a promising biobased building-block for monomer synthesis. Green Chem 16:1987–1998. https://doi.org/10.1039/C3GC42613K
Ananda SA, Bernard W, Ashfaqur R (2012) Vanillin based polymers: I. An electrochemical route to polyvanillin. Green Chem 14:2395–2397. https://doi.org/10.1039/C2GC35645G
Abdelaty MSA, Kuckling D (2016) Synthesis and characterization of new functional photo cross-linkable smart polymers containing vanillin derivatives. Gels 2:1–13. https://doi.org/10.3390/gels2010003
Firdaus M, Meier AR (2013) Renewable copolymers derived from vanillin and fatty acid derivatives. Eur Polym J 49:156–166. https://doi.org/10.1016/j.eurpolymj.2012.10.017
Abdelaty MSA (2018) Preparation and characterization of environmental functional poly(styrene-co-2-[(diethylamino)methyl]-4-formyl-6-methoxy-phenyl acrylate) copolymers for amino acid post polymerization. Open J Polym Chem 8:41–55. https://doi.org/10.4236/ojpchem.2018.83005
Abdelaty MSA (2018) Poly(N-isopropylacrylamide-co-2-((diethylamino)methyl)-4 formyl-6-methoxyphenylacrylate) environmental functional copolymers: synthesis, characterizations, and grafting with amino acids. Biomolecules 8:138. https://doi.org/10.3390/biom8040138
Kuckling D, Adler H-JP, Arndt K-F, Ling L, Habicher WD (2000) Temperature and pH dependent solubility of novel poly(N-isopropylacrylamide) copolymers. Macromol Chem Phys 201:273–280
Acknowledgements
We would like to thank the University of Paderborn.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelaty, M.S.A. Trends in the Phase Separation Temperature Optimization of a Functional and Thermo-pH Responsive Terpolymer of Poly (N-isopropylacrylamide-co-N-(2-(dimethylamino)ethyl) Acrylamide-co-vanillin Acrylate). J Polym Environ 29, 3116–3129 (2021). https://doi.org/10.1007/s10924-021-02096-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-021-02096-4