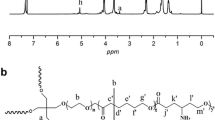
Abstract
To prepare amphiphilic block copolymers consisting of biocompatible and biodegradable segments, herein we report synthesis of diblock copolymers having ε-caprolactone (ε-CL) repeating units in one block and amino acid-based acrylate monomers in another segment. The block copolymers were prepared by a combination of metal-free ring-opening polymerization (ROP) of ε-CL and reversible addition-fragmentation chain transfer (RAFT) polymerization of tert-butyloxycarbonyl (Boc)-alanine/Boc-leucine based acrylate monomers. The ROP of ε-CL was initiated with diphenyl phosphate (DPP) as a metal-free catalyst in conjunction with a heterofunctional initiator, benzyl-2-hydroxyethyl carbonotrithioate, produced trithiocarbonate terminated poly(ε-caprolactone) (PCL). This was further employed as macro-chain transfer agent for the synthesis of side chain amino acid containing block via RAFT. Deprotection of Boc group pendants from the block copolymers under acidic conditions at room temperature provided pH responsive block copolymers with positively charged cationic primary amine functionalities. Furthermore, self-assembling nature of these block copolymers in aqueous medium was examined through dynamic light scattering (DLS) and field emission-scanning electron microscopy (FE-SEM).
Graphic Abstract









Similar content being viewed by others
References
Basterretxea A, Jehanno C, Mecerreyes D, Sardon H ( 2019) Dual organocatalysts based on ionic mixtures of acids and bases: a step toward high temperature polymerizations. ACS Macro Lett 8:1055–1062
Cha Y, Jarrett-Wilkins C, Rahman MdA, Zhu T, Sha Y, Manners I, Tang C (2019) Crystallization-driven self-assembly of metallo-polyelectrolyte block copolymers with a polycaprolactone core-forming segment. ACS Macro Lett. 8:835–840
Clément B, Grignard B, Koole L, Jérôme C, Lecomte P (2012) Metal-free strategies for the synthesis of functional and well-defined polyphosphoesters. Macromolecules 45:4476–4486
Brannigan RP, Dove AP (2017) Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 5:9–21
Jehanno C, Mezzasalma L, Sardon H, Ruipérez F, Coulembier O, Taton D (2019) Benzoic acid as an efficient organocatalyst for the statistical ring-opening co-polymerization of ε-caprolactone and l-lactide: a computational investigation. Macromolecules 52:9238–9247
Ren JM, Fu Q, Blencowe A, Qiao GG (2012) Organic catalyst-mediated ring-opening polymerization for the highly efficient synthesis of polyester-based star polymers. ACS Macro Lett. 1:681–686
Bouyahyi M, Duchateau R (2014) Metal-based catalysts for controlled ring-opening polymerization of macrolactones: high molecular weight and well-Defined copolymer architectures. Macromolecules. 2:517–524
Zhu N, Zhang Z, Feng W, Zeng Y, Li Z, Fang Z, Zhang K, Li Z, Guo K (2015) Sn(OTf)2 catalyzed continuous flow ring-opening polymerization of ε-caprolactone. RSC Adv. 5:31554–31557
Wang Q, Li H, Wei Q, Sun JZ, Wang J, Zhang XA, Qin A, Tang BZ (2013) Metal-free click polymerizations of activated azide and alkynes. Polym. Chem 4:1396–1401
Brannigan RP, Walder A, Dove AP (2014) Block copolymer materials from the organocatalytic ring-opening polymerization of a pentaerythritol-derived cyclic carbonate. J Polym Sci Part A: Polym Chem 52:2279–2286
Higuchi M, Kanazawa A, Aoshima S (2020) Tandem unzipping and scrambling reactions for the synthesis of alternating copolymers by the cationic ring-opening copolymerization of a cyclic acetal and a cyclic ester. ACS Macro Lett. 9:77–83
Clement B, Grignard B, Koole L, Jerome C, Lecomte P (2012) Metal-free strategies for the synthesis of functional and well-defined polyphosphoesters. Macromolecules 45:4476–4486
Li X, Li H, Zhao Y, Tang X, Ma S, Gong B, Li M (2015) Facile synthesis of well-defined hydrophilic polyesters as degradable poly(ethylene glycol)-like biomaterials. Polym Chem 6:6452–6456
Danko M, Basko M, Ďurkáčová S, Duda A, Mosnáček J (2018) Functional polyesters with pendant double bonds prepared by coordination-insertion and cationic ring-opening copolymerizations of ε-caprolactone with renewable tulipalin A. Macromolecules 51:3582–3596
Ercole F, Rodda AE, Meagher L, Forsythe JS, Dove AP (2014) Surface grafted poly(ε-caprolactone) prepared using organocatalysed ring-opening polymerisation followed by SI-ATRP. Polym. Chem. 5:2809–2815
Zhao J, Pahovnik D, Gnanou Y, Hadjichristidis N (2014) Phosphazene-promoted metal-free ring-opening polymerization of ethylene oxide initiated by carboxylic acid. Macromolecules 47:1693–1698
Alamri H, Zhao J, Pahovnik D, Hadjichristidis N (2014) Phosphazene-catalyzed ring-opening polymerization of ε-caprolactone: influence of solvents and initiators. Polym. Chem. 5:5471–5478
Coady J, Horn HW, Jones GO, Sardon H, Engler AC, Waymouth RM, Rice JE, Yang YY, Hedrick JL (2013) Polymerizing base sensitive cyclic carbonates using acid catalysis. ACS Macro Lett. 2:306–312
Deng Y, Zou T, Tao X, Semetey V, Trepout S, Marco S, Ling J, Li MH (2015) Poly(ε-caprolactone)-block-polysarcosine by ring-opening polymerization of sarcosine N-thiocarboxyanhydride: synthesis and thermoresponsive self-assembly. Biomacromolecules 16:3265–3274
Jose L, Hwang AR, Lee C, Shim K, Song JK, Soo S, An A, Paika H (2020) Nitrilotriacetic acid-end-functionalized polycaprolactone as a template for polymer–protein nanocarriers. Polym Chem 11:1580–1588
Delplace V, Nicolas J (2015) Degradable vinyl polymers for biomedical applications. Nat. Chem. 7:771–784
Wang S, Kesava SV, Gomez ED, Robertson ML (2013) Sustainable thermoplastic elastomers derived from fatty acids. Macromolecules 46:7202–7212
Kryuchkov MA, Christophe Detrembleur C, Bazuin G (2014) Linear amphiphilicdiblock copolymers of lactide and 2-dimethylaminoethyl methacrylate using bifunctional-initiator and one-pot approaches. Polymer 55:2316–2324
Lenoir S, Pagnoulle C, Detrembleur C, Galleni M, Jerome R (2006) New antibacterial cationic surfactants prepared by atom transfer radical polymerization. J. Polym. Sci. Part A: Polym. Chem. 44:1214–1224
Bauri K, De P, Shah PN, Li R, Faust R (2013) Polyisobutylene-based helical block copolymers with pH-responsive cationic side-Chain amino acid moieties by tandem living polymerizations. Macromolecules 46:5861–5870
Schmid C, Weidner S, Falkenhagen J, Barner-Kowollik C (2012) In-depth LCCC-(GELC)-SEC characterization of ABA block copolymers generated by a mechanistic switch from RAFT to ROP. Macromolecules 45:87–99
Mishra AK, Patel VK, Vishwakarma NK, Biswas CS, Raula M, Misra A, Mandal TK, Ray B (2011) Synthesis of well-defined amphiphilicpoly(ε-caprolactone)-b-poly(N-vinylpyrrolidone) block Copolymers via the combination of ROP and xanthate-mediated RAFT polymerization. Macromolecules 44:2465–2473
Kang HU, Yu YC, Shin SJ, Kim J, Youk JH (2013) One-Pot synthesis of poly(N-vinylpyrrolidone)-b-poly(ε-caprolactone) block copolymers using a dual initiator for RAFT polymerization and ROP. Macromolecules 46:1291–1295
de Freitas AGO, Trindade SG, Muraro PIR, Schmidt V, Satti AJ, Villar MA, Ciolino AE, Giacomelli C (2013) Controlled One-pot synthesis of polystyrene-block-polycaprolactone copolymers by simultaneous RAFT and ROP. Macromol Chem Phys 214:2336–2344
Scarano W, de Souza P, Stenzel MH (2015) Dual-drug delivery of curcumin and platinum drugs in polymeric micelles enhances the synergistic effects: a double act for the treatment of multidrug-resistant cancer. Biomater Sci 3:163–174
Roy SG, De P (2014) pH responsive polymers with amino acids in the side chains and their potential applications. J Appl Polym Sci 131:41084
Bauri K, Roy SG, De P (2016) Sidechain aminoacidderived cationic chiral polymers by controlled radical polymerization. Macromol Chem Phys 217:365–379
Sun H, Gao C (2010) Facile Synthesis of multiamino vinyl poly(amino acid)s for promising bioapplications. Biomacromolecules 11:3609–3616
Kumar S, Acharya R, Chatterji U, De P (2013) Controlled synthesis of pH responsive cationic polymers containing side-chain peptide moieties via RAFT polymerization and their self-assembly. J Mater Chem B 1:946–957
Bauri K, Roy SG, Pant S, De P (2013) Controlled synthesis of amino acid-based pH-responsive chiral polymers and self-assembly of their block copolymers. Langmuir 29:2764–2774
Roy SG, Acharya R, Chatterji U, De P (2013) RAFT polymerization of methacrylates containing a tryptophan moiety: controlled synthesis of biocompatible fluorescent cationic chiral polymers with smart pH-responsiveness. Polym Chem 4:1141–1152
Bauri K, Nandi M, De P (2018) Amino acid-derived stimuli-responsive polymers and their applications. Polym Chem 9:1257–1287
Skey J, O’Reilly RK (2008) Facile one pot synthesis of a range of reversible addition–fragmentation chain transfer (RAFT) agents, Chem Commun 4183–4185
Makiguchi K, Satoh T, Kakuchi T (2011) Diphenylphosphate as an efficient cationic organocatalyst for controlled/living ring-opening polymerization of δ-valerolactone and ε-caprolactone. Macromolecules 44:1999–2005
Batiste C, Meyersohn MS, Watts A, Hillmyer MA (2020) Efficient polymerization of methyl-ε-caprolactone mixtures to access sustainable aliphatic polyesters. Macromolecules 53:1795–1808
Zhai S, Ma Y, Chen Y, Li D, Cao J, Liu Y, Cai M, Xie X, Chen Y, Luo X (2014) Synthesis of an amphiphilic block copolymer containing zwitterionicsulfobetaine as a novel pH-sensitive drug carrier. Polym Chem 5:1285–1297
Shimoboji T, Ding ZL, Stayton PS, Hoffman AS (2002) Photoswitching of ligand association with a photoresponsive polymer−protein conjugate. Bioconjug Chem 13:915–919
Haldar U, Nandi M, Ruidas B, De P (2015) Controlled synthesis of amino-acid based tadpole-shaped organic/inorganic hybrid polymers and their self-assembly in aqueous media. Euro Polym J 67:274–283
Saha A, Paira TK, Biswas M, Jana S, Banerjee S, Mandal TK (2015) Combined atom-transfer radical polymerization and ring-opening polymerization to design polymer–polypeptide copolymer conjugates toward self-aggregated hybrid micro/nanospheres for dye encapsulation. J Polym Sci Part A: Polym Chem 53:2313–2319
Li M, Song W, Tang Z, Lv S, Lin L, Sun H, Li Q, Yang Y, Hong H, Chen X (2013) Nanoscaledpoly(l-glutamic acid)/doxorubicin-amphiphilecomplex as pH-responsive drug delivery system for effective treatment of non small cell lung cancer. ACS Appl Mater Interfaces 5:1781–1792
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azmeera, V., Haldar, U., Roy, S.G. et al. Block Copolymers of Poly(ε-caprolactone) with pH-Responsive Side-Chain Amino Acid Moieties. J Polym Environ 29, 209–218 (2021). https://doi.org/10.1007/s10924-020-01872-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01872-y