Abstract
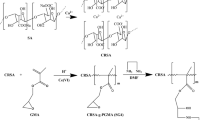
In the present study, adsorptive removal of rhodamin-B (RMN-B) from aqueous solution was investigated by using nano Zn–Al–Fe3O4 incorporated sodium alginate (SA) beads. SA is a natural polysaccharide, non toxic and biodegradable material. The incorporation of Zn–Al–Fe3O4 into SA was done by ionic polymerization method using CaCl2. A batch mode study was used for the adsorption of RMN-B onto Zn–Al–Fe3O4/ alginate beads. The adsorption efficiency of RMN-B was investigated by the following modeling equations like Freundlich, Langmuir, Pseudo-first order, Pseudo-second order, Intraparticle diffusion, Gibbs free energy, Enthalpy and Entropy. The predicted experimental results of various modeling equations were implied that the process follow Langmuir and pseudo-second order equations. The intraparticle diffusion parameters indicate that the diffusion between RMN-B and nano beads were predominant in throughout of process. The surface morphology of nano beads of before and after adsorption were show that RMN-B is strongly adhered on whole surface of the nano material. The FTIR spectrum indicates that the RMN-B strongly adsorbed on the surface and TGA study reveals that the alginate/Zn–Al–Fe3O4/Ca material has an excellent temperature withstand capacity.





Similar content being viewed by others
References
Ngomsik AF, Bee A, Draye M, Cote G, Cabuil V (2005) Magnetic nano and micro particles for metal removal and environmental applications: a review. C R Chimie 8:963–970
Zhou L, Gao C, Xu WJ (2010) Magnetic dendristic materials for highly efficient adsorption of dyes and drugs. ACS Appl Mater Interfaces 2:1483–1491
Yadav M, Rhee KY (2012) Superabsorbent nano (alginate-g-PAMPS/MMT): Synthesis, characterization and swelling behavior. Carbohydrate Poly 90:165–173
Zhang F, Yin X, Lan J, Zhang W (2016) Application of Ba3(PO4)2/Fe3O4 as a novel magnetic adsorbent to remove methyl blue from aqueous solution. J Mater Sci 51:3525–3535
Ignat M, Samoila P, Cojocaru C, Sacarescu L (2016) Valeria harabagiu novel synthesis route for chitosan-coated zinc ferrite nanoparticles as potential sorbents for wastewater treatment. Chem Eng Commun 203(12):1591–1599
Jiqing J, Tang WQiu,J, Chen L, Jing L (2016) Synthesis of well-defined Fe3O4 nanorods/N-doped graphene for lithium-ion batteries. Nano Res 9(5):1256–1266
Dehghani MH, Mohammadi M, Mohammadi MA, Mahvi AH, Yetilmezsoy K, Bhatnagar A (2016) Behzad Heibati, Gordon McKay, equilibrium and kinetic studies of trihalomethanes adsorption onto multi-walled carbon nanotubes. Water Air Soil Pollut 227:332
Feng J, Wu NSun,D, Yang H, Xu H, Wei Yan (2016) Preparation of Fe3O4/TiO2/polypyrrole ternary magnetic composite and using as adsorbent for the removal of acid red G. J Polym Environ. https://doi.org/10.1007/s10924-016-0839-7
Ai L, Li M, Li L (2011) Adsorption of RMN-B from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: kinetics, isotherms, and thermodynamics. J Chem Eng Data 56(8):3475–3483
Mahmoodi NM (2013) Magnetic ferrite nanoparticle-alginate composite: synthesis, characterization and binary system dye removal. J Taiwan Institute of Chem Eng 44:322–330
Kumar M, Tamilarasan R, Sivakumar V (2013) Adsorption of Victoria blue by carbon/Ba/alginate beads: kinetics, thermodynamics and isotherm studies. Carbohydr Polym 98:505–513
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater-a review. Sci Total Environ 333:37–58
Neill CO, Hawkes FR, Hawkes DL, Lourenco ND, Pinheiro HM, Delee W (1999) Colour in textile effluents-sources, measurement, discharge consents and simulation: a review. J Chem Tech Biotech 74:1009–1018
Ahmed Awadallah F (2016) Adsorptive removal of malachite green chloride and reactive red-198 from aqueous solutions by using multiwall carbon nanotubes-graft-Poly (2-acrylamido-2-methyl-1-propanesulfonic acid). J Polym Environ. https://doi.org/10.1007/s10924-016-0804-5
Vaidyanathan G, Sendhilnathan S (2008) Characterization of Co1–xZnxFe2O4 nanoparticles synthesized by co-precipitation method. Phys B 403:2157–2167
Saraswathy A, Nazeer SS, Nimi N, Arumugam S, Shenoy SJ, Jayasree RS (2014) Synthesis and characterization of dextran stabilized superparamagnetic iron oxide nanoparticles for in vivo MR imaging of liver fibrosis. Carbohydr Polym 101:760–768
Deans JR, Dixon BG (1992) Uptake of Pb2+ and Cu2+ by novel alginates. Water Res 26:469–472
Yamuna RT, Namasivayam C (1994) Dye removal from wastewater by adsorption on ‘waste’ Fe (III)/Cr (III) hydroxide. Waste Manag 14:643–648
Arshadi M, Salimi Vahid F, Salvacion JWL, Soleymanzadeh M (2013) A practical organometallic decorated nano-size SiO2–Al2O3 mixed-oxides for methyl orange orange removal from aqueous solution. Appl Surface Sci 280:726–736
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Sohrabnezhad S, Pourahmad A (2010) Comparison absorption of new RMN-B dye in zeolite and nanocrystal zeolite. Desalination 256:84–89
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soci 40:1361–1403
Lagergren S, About the theory of so called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens. 24 (1898) 1–39
Alkan M, Dogan M, Turhan Y, Demirbas O, Turan P (2008) Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions. Chem Eng J 139:213–223
Hameed BH, Tan IAW, Ahmad AL (2008) Adsorption isotherm, kinetic modeling and mechanism of 2, 4, 6-trichlorophenol on coconut husk-based activated carbon. Chem Eng J 144:235–244
Malash GF, Khaiary MIE (2010) Piecewise linear regression: A statistical method for the analysis of experimental adsorption data by the intraparticle-diffusion models. Chem Eng J 163:256–263
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour Technol 98:2897–2904
Kumar M, Tamilarasan R (2013) Modeling studies: adsorption of aniline blue by using Prosopis Juliflora carbon/Ca/alginate polymer composite beads. Carbohydr Polym 92:2171–2180
Smith JM, Van Ness HC (1987) Introduction to chemical engineering thermodynamics. McGraw-Hill, Singapore
Latif Aref AH, Navarchian D, Dadkhah (2016) Adsorption of crystal violet dye from aqueous solution by poly(acrylamide-co-maleic acid)/montmorillonite nanocomposite. J Polym Environ. https://doi.org/10.1007/s10924-016-0842-z
Bidyadhar M, Samit Kumar R (2013) Synthesis of interpenetrating network hydrogel from poly(acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr Polym 98:257–269
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, M., Vijayakumar, G. & Tamilarasan, R. Synthesis, Characterization and Experimental Studies of Nano Zn–Al–Fe3O4 Blended Alginate/Ca Beads for the Adsorption of Rhodamin B. J Polym Environ 27, 106–117 (2019). https://doi.org/10.1007/s10924-018-1318-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1318-0