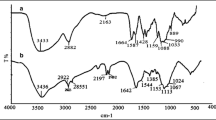
Abstract
The precipitation technique of Mn-IV/itaconic acid redox pair was chosen for grafting meth acrylic acid onto chitosan. This was done to maximize the graft yield and minimize the homopolymer formation to lower extent in addition to prepare water soluble chitosan instead of non-eco friendly acid soluble one. Evidence of grafting was confirmed instrumentally by (FTIR, SEM, and TGA), titrimetrically using carboxyl content and gravimetrically using dry weight method. The mechanism of grafting and factors affected grafting reaction were studied in details, and the optimum reaction conditions were obtained at [chitosan] 2 g, [MnO2] 25.9 mmol/100 g sample, [itaconic acid],100 mmol/L, [MAA] 50% bows,[liquor ratio], 25, reaction time, 2 h, and [reaction temperature], 65 °C. It is also seen from the obtained results that, the resultant copolymer showed the following findings: (a) only nitric acid solutions are proved to be a superior solvent for the grafted product; (b) superabsorbent towards water (197 g H2O/g polymer) and saline absorbency (60 g saline solution/g polymer) after cross-linked with epichlorohydrin, (c) higher water solubility (21%) at 48% graft yield and (d) 73.5% copper ion removal in comparison with 41% for chitosan.
Similar content being viewed by others
Introduction
Chitosan as one of the most abundant natural carbohydrate polymers world-wide after cellulose is being considered as the N-deacetylated form of chitin that is predominantly found in the exo-skeleton of crustaceans [1]. It has received much consideration in many areas due to its biodegradability, adaptability, biocompatibility, non-toxicity and antimicrobial properties [2, 3]. But it suffers from some serious drawback the most outstanding are poor solubility in water and high fragility that need development to increase its manipulation especially for environmental concerns [4]. So, a series of chemical modifications onto chitosan have been conducted, like nitration, phosphorylation and sulphation in addition to the graft copolymerization reaction which consider as one of the most fascinating field for molecular designs that lead to unique types of hybrid materials and introduce a desired properties that broaden its application range via selecting several types of side chains without sacrificing its biodegradable nature [5,6,7,8]. Indeed, grafting onto chitosan is a common way to improve its properties like chelation [9] or complexation properties [10], bacteriostatic effect [11] or enhancing adsorption properties [12, 13]. Moreover, it is well known that chitosan is considered as highly basic biopolymer due to presence of amine groups at the C2 position in comparison with other biopolymers like starch, cellulose, dextrin, alginic acid and pectin, which are either neutral or acidic in nature. Also, the high degree of functionality of chitosan i.e. the presence of two hydroxyl groups (primary at C3 and secondary at C6 positions respectively) and one primary amine group per each repeated unit allows its further modifications with a wide range of reactants [4, 8]. Recently, a number of classic initiator systems such as, potassium persulfate, ceric ammonium nitrate, ammonium persulfate, potassium bromate, potassium diperiodatocuprate(III), 2,2-azobisisobutyronitrile, ferrous ammonium sulfate and potassium permanganate alone or in presence of acids have been used to initiate chitosan grafting with different vinyl monomers such as vinyl acetate, acrylonitrile, acrylic acid, meth acrylic acids and methyl methacrylate for particular end use [14,15,16,17,18,19,20]. Among the above initiators used, ceric ammonium nitrate is the most efficient one, but it leads to high homopolymer formation, lower graft yield and high cost. So, our research team directed to solve the above problems by choosing our precipitated technique namely Mn-IV in presence of itaconic acid redox pair for graft copolymerization onto chitosan to maximize the graft yield and grafting reaction efficiency in addition to lower homopolymer formation to lower extent as our first targeted aim. Beside, to our knowledge until now, the above initiation tool used here, are not cited in the literature to initiate graft copolymerization onto chitosan. On the other hand, choosing MAA as a the most frequently reactive vinyl monomers for grafting onto chitosan due to its high grafting efficiency, availability and hydrophilicity as well as introducing several smart characteristics including superabsorbent hydrogel, metal ion chelation and water solubility especially with respect to the environmental concerns as our second targeted aim [21, 22]. Hence, the aim of this study was undertaken with graft copolymerization onto chitosan backbone by MAA using Mn-IV/itaconic acid redox pair and the resultant copolymer was characterized by Fourier transforms infrared spectroscopy (FTIR), scanning electron microscopy (SEM), TGA, carboxyl content and solubility to elucidate the structure changes in comparison with native chitosan. Factors affecting the graft copolymerization were studied in details and the mechanism of the grafting was anticipated. Furthermore, the solubility of the graft copolymers in different organic and inorganic solvents in addition to water was investigated to see the appropriate solvent for the grafted products. Finally, application of poly(MAA)–chitosan graft copolymers having different graft yields for water and saline absorbency and copper ion removal were also studied.
Experimental Part
Materials
Chitosan [α-(1–4)2-amino 2-deoxyβ-d-glucan], whose degree of deacetylation was 90.5% and apparent viscosity was 281 centipoises, was obtained from Yuhuan Biochemical Co. (China). Meth acrylic acid stabilized with 0.01% hydroquinone, was freshly distilled at 75 °C and pressure of 100 mm Hg. It was stored at − 10 °C until used. Potassium permanganate, sodium hydroxide, epichlorohydrin, sodium chloride, itaconic, formic, acetic, malic, phosphorous and nitric acids, ethyl alcohol, acetone, and DMF and copper sulphate 6 H2O were of technical grade chemicals used throughout the experiments.
Grafting Process
Pretreatment of Chitosan with Potassium Permanganate
An accurate weight of chitosan was immersed in 100 mL aqueous KMnO4 solution (0.025–0.2 M) for 30 min at 50 °C in a conical flask with a continuous shaking to ensure the homogeneous deposition of MnO2 all over the chitosan sample. After this treatment the chitosan sample was washed thoroughly with second distilled water to remove excess KMnO4 solution. It was then squeezed between two filter papers before being introduced into the polymerization solution.
Determination of MnO2 Quantity onto Chitosan
The amount of MnO2 deposited onto the chitosan sample was determined by adding 10 mL 0.1 M oxalic acid and 10 mL 2 M sulphuric acids to the chitosan sample treated with permanganate in a conical flask. The mixture was gently heated to about (65 ± 0.5 °C) and then titrated against a diluted solution of 0.01 M KMnO4.
where V is the volume of KMnO4 (ml) equivalent to the amount of MnO2 in the chitosan multiplied by normality of oxalic acid (0.1 N) and W is the weight of the sample used (2 g).
Grafting Procedure
Unless otherwise indicated, the desired amount of potassium permanganate treated chitosan samples (0.5–4 g) was dissolved in 30% acetic acid solution (1% concentration) based on the total volume of water at room temperature and left overnight in a close glass vessel with continuous stirring to insure the complete dissolution of chitosan. Then different concentrations of itaconic acid (20–150 mmol/L) and meth acrylic acid (25–150% based on weight of chitosan) were then added. The flask content was kept in a thermostatic water bath at different temperatures (45–75 °C) for different time interval (15–180 min). Liquor ratio ranged from 5 to 50 (i.e. total volume of water depending on chitosan weight used) was used and nitrogen gas was purged into the reaction mixture to avoid the presence of oxygen and the flask contents were shaken frequently during the course of reaction. After the desired reaction time, the grafted chitosan samples were separated by neutralization of excess acetic acid with dilute sodium hydroxide solution, filtered, and then washed with distilled water till pH 7. The sample was then squeezed between two filter papers and dried in an electric oven at 60 °C for 4 h and cooled over P2O5 in a vacuum desecrator overnight.
Poly(MAA)–chitosan graft copolymers having different graft yields were synthesized by keeping the entire grafting reaction conditions constant and changing only the amount of MAA concentration.
Homopolymer Removal
At the end of the reaction, the flask contents were poured over 100 mL of ethyl alcohol where a precipitate was formed, which consisted of poly(MAA)–chitosan graft copolymer and poly(MAA) (homopolymer). The latter can be removed completely by washing with mixture of ethyl alcohol:water mixture (50:50) several times (for 25 min each) at 50 °C, filtered, and finally dried in an electric oven at 65◦C for 5 h. It was found experimentally that washing 2–3 times with the above mixture is quite enough for complete homopolymer removal in physical mixtures of chitosan/poly(MAA) by tracing the carboxyl content [23] of these mixtures after each wash until constant value.
Estimation of Graft Yield (%)
Gravimetric Method [8]
The graft yield (%) was assessed gravimetrically using dry-weight based tool. This was determined by keeping the grafted products at 65 °C for a period of 4 h in a weighing bottle over P2O5 in vacuum desiccators until a constant weight was obtained as shown below:
where W0 and W1 are the weight of chitosan and grafted chitosan (g) respectively.
Titrimetric Method (Carboxyl Content)
The graft yield (%) was estimated titrimetrically by tracing the carboxyl content of the grafted sample via acid base titration and the graft yield (%) was calculated as follows:
where 0.86 is the molecular weight of meth acrylic acid divided by 100.
This was done to assess the difference between the degree of metrological precision when calculating the graft yield (%) values of the grafted sample either gravimetrically or titrimetrically.
Proof of Grafting
To confirm that the MAA grafted onto chitosan, the poly(MAA)–chitosan graft copolymer and blank chitosan as a control were characterized using FTIR, SEM, TGA, carboxyl content and water solubility.
Fourier Transforms Infrared Spectroscopy (FTIR)
FTIR was recorded on a Nicolet 380 spectrophotometer (Thermo Scientific), with a spectral range 4000–400 cm−1, a resolution of 4 cm−1 and a number of scan 32.
Scanning Electron Microscopy (SEM)
SEM images for the samples were taken using (Joel GM4200, Quanta 200, Holland). The surfaces of all the samples were coated with a gold thin layer under vacuum before SEM studies at an accelerating voltage of 20 kV.
Thermal Analysis
The TGA analysis was carried out on TGA50 (TA Shimadzu, Inc.) at a temperature range from room temperature to 800 °C at a heating rate of 10 °C min−1 under N2 atmosphere.
Solubility Test
Acids and Solvents
Eight solvents viz. (formic, acetic, malic, phosphorous, and nitric acids in addition to DMF, acetone and ethanol) were selected to examine their suitability for dissolving (dissolution) the resultant grafted product including native chitosan, poly(MAA) (homopolymer) and poly(MAA)–chitosan graft copolymer having (G.Y. 44.9%). This was done via visual assessment of the solution. All acids used either organic or inorganic were used at 1% (v/v). While other solvents were used as they received.
Water Solubility (%) [24]
The solubility percentage of tested samples in water was estimated gravimetrically by treating 1 g of sample in 50 mL of water at 70 °C for 2 h. The sample was filtered, and filtrate was transferred into a Petri dish and was dried in a ventilating oven till constant weight was obtained.
Water and Saline Absorbency (Filtration Method)
The application of poly(MAA)–chitosan graft copolymer (21% solubility) in water and saline absorbency required first to crosslink the above copolymer with epichlorohdrin to prevent its water solubility as shown below:
Poly(MAA)–chitosan graft copolymer was dissolved in 1% (v/v) acetic acid solution to produce a viscous solution with 1% (w/v) chitosan. An aliquot of 10 mL of 12.5 mol/L epichlorohydrin was added to the grafted chitosan solution and kept at 60 °C for 2 h. The grafted chitosan–epichlorohydrin molar ratio used was 1.0/0.5. Subsequently, 50 mL of 0.1 mol/L sodium hydroxide was added and the system was boiled for 3 h. The grafted chitosan–epichlorohydrin sample was filtered and washed with double distilled water to remove the excess of crosslinking agents and then dried in electric oven at 65 °C for 5 h. Then an accurate dry weight of cross-linked poly(MAA)–chitosan graft copolymer (1.0 g) was immersed in 1000 mL distilled water for 30 min and poured into a 40 mesh sieve of known weight. Water was allowed to drain for 1 h, then, the hydrated sample and the sieve were weighted and the absorbency was calculated as follows:
where W is the weight of dry sample, W1, is the weight of the sieve, and W2, the weight of the swollen sample plus the weight of the sieve. A similar technique was used to study saline solution absorbency, where the dry sample was immersed in 100 mL of saline solution (0.9% NaCl) for 30 min.
The water absorbency was determined three times for each sample and its value was expressed in terms of ± standard deviation (SD) to achieve the appropriate precision measured values.
Copper Ions Removal
An aqueous solution of copper ions with a fixed concentration (Cu 203 ppm) was prepared by dissolving 0.8 g cupric sulphate (CuSO4·5H2O,) in 1 L double distilled water. Then chitosan and poly(MAA)–chitosan graft copolymers (0.5–4.0% w/v) were added to (100 mL) of the above solution, and the dispersion was stirred for 25 min at room temperature to form a polymer copper ion complex. The latter was then separated by filtration and the filtrate was used for the residual metal analysis using atomic absorption spectrophotometer.
Statistical Analysis
All experiments were carried out in triplicate, and average values with standard deviation are reported. Our instruments are calibrated using our primary standard apparatus and/or certified reference materials to insure their metrological precision all over the measurement process.
Results and Discussion
As we have before observed, the rate and extent of grafting of synthetic vinyl monomers onto carbohydrate polymers backbone are very sensitive towards varying the reaction conditions [25,26,27,28,29,30,31,32,33]. So, we start with a systematic study on the effect of changing the reaction variables on grafting of methacrylic acid onto chitosan using Mn-IV/itaconic acid as a novel redox pair with the aim of preparing water soluble grafted products having higher graft yield and lower formation of homopolymer.
Effect of Potassium Permanganate Concentration on the Amount of MnO2 Deposited onto Chitosan
The chitosan samples treated with KMnO4 were examined for MnO2 deposited over them as designated in the experimental section. Table 1 displays the relation between the amount of MnO2 deposited onto the chitosan sample and the KMnO4 concentration. The obtained data reveals that the amount of MnO2 deposit increases by increasing the KMnO4 concentration up to 0.15 M. Beyond this concentration no marked increase was observed and the amount of MnO2 deposited is the same all over the studied range of KMnO4 concentration. It is sensible to undertake that MnO2 particles are deposited constantly all over the chitosan samples and are found most probably at the hydroxyl groups of chitosan. Once the accessible hydroxyl groups are full with MnO2 particles, additional deposition of MnO2 will be hard and, if deposited, the particles will be easily removed during the repeating washing processes. This would elucidate why the quantity of MnO2 deposited over the chitosan sample remains constant at higher KMnO4 concentrations, particularly above 0.15 M.
Grafting Mechanism
Native chitosan was turned to a brownish, dark brownish or even black color when it was treated with KMnO4 solution, depending on the concentration of the KMnO4 solution used as described above. In the presence of itaconic acid (HA), primary radical species formation occurs as a result of the action of an acid on the MnO2 deposited [34, 35] as shown in Scheme 1.
Once the carboxylate free radical species ·COO– (A·) are formed, they produce chitosan macro radicals via direct abstraction of hydrogen atom either from hydroxyl groups (Eq. 1) and/or amine groups (Eq. 2) from chitosan molecules [8].
where CS–OH and CS–NH2 represent native chitosan while, CSO· and CSNH· represent chitosan macro radicals or CS· for simplicity.
Chitosan macro radicals may also be formed by direct attack of Mn4+ or Mn3+ ions on the chitosan molecule via abstraction of the hydrogen atom either from hydroxyl or amine groups as described below in Eqs. 3 and 4.
In presence of MAA the chitosan macro radicals is added to the double bond of MAA, resulting in a covalent bond between MAA and chitosan with formation of free radical on the monomer. i.e. a chain is initiated. Successive addition of MAA molecules to the initiated chain propagates the grafting reaction onto chitosan as described below in Eqs. 5 and 6.


Finally, termination of the growing grafted chain may occur via reaction with the initiator (·R), coupling and disproportionation as mention below by Eqs. 7–9.



Effect of MnO2 Deposited on the Graft Yield (%)
Figure 1 display the graft yield of poly(MAA)–chitosan graft copolymers versus the amount of MnO2 deposited onto chitosan. Clearly, the graft yields increases extensively as the MnO2 deposit increases until MnO2 concentration of 25.9 mmol/100 g sample is reached and then remains constant. The improvement in grafting by increasing the amount of MnO2 deposited up to the above value is duo to MnO2 and free radical species contribute predominantly in the formation of chitosan macro radicals, which are capable of initiating grafting. On the other hand, the fact that the graft yield remains constant after 25.9 mmol/100 g sample is deposited onto the chitosan may be recognized to: (a) a faster termination rate between two chain radicals via bimolecular collision, (b) a lower diffusion rate of MAA from the aqueous phase to the chitosan phase duo to the excess amount of MnO2 deposited and (c) a higher oxygen production of inhibiting oxygen at a higher MnO2 concentration duo to the side reaction designated below.
Effect of Itaconic Acid Concentration
The effect of itaconic acid concentrations on the graft yield of poly(MAA)–chitosan graft copolymers are shown in Fig. 2. Obviously, increasing itaconic acid concentration up to 100 mmol/L is accompanied by improvement in the graft yield and above this concentration, grafting decreases. The enhancement in grafting may be due to an increase in the reactive species concentration that results in high formation of primary free radicals to react with other ingredients rather than chitosan. On the other hand, the decrease in grafting above 100 mmol/L itaconic acid concentration could be elucidated in terms of increasing (a) the coagulation of homopolymer in the solution and in the chitosan structure at lower pH values, which impedes the diffusion of MAA and initiator into the chitosan structure, and (b) a higher oxygen production of inhibiting oxygen at a higher MnO2 concentration as shown in Sect. 3.2.
Effect of Monomer Concentration
The influence of MAA concentrations on the graft yield of poly(MAA)–chitosan graft copolymers are shown in Fig. 3. It was seen from Fig. 3 that, as the concentration of MAA increases, the graft yield also increases within the range studied. This could be due to the larger accessibility of monomer moieties in the close proximity to the chitosan macro radicals; which are relatively immobile and for grafting to occur, the monomer molecules need to be in close proximity to the chitosan.
Effect Liquor Ratio
The impact of liquor ratio used on the graft yield of poly(MAA)–chitosan graft copolymers are shown in Fig. 4. The amounts of all other ingredients were kept constant in this case. It is seen Fig. 4 that, increasing the liquor ratio up to 25 brings about augmentation in grafting, then decrease by increasing liquor ratio up to 50. So, it is rationally to say that, of all liquor ratio studied, a liquor ratio 25 constitute the best. This fact can be explained by diffusion controlled phenomena, at which at this explicit liquor ratio a good grafting environment is produced through close association of the monomer and initiator with the chitosan macromolecules. Once this is the case, larger accessibility of the monomer and initiator in the vicinity of the chitosan molecules occurs, thereby leading to higher grafting. The opposite hold truth at higher liquor ratio.
Chitosan Concentration
Graft yield (%) of poly(MAA)–chitosan graft copolymer increases by increasing the concentration of chitosan within the range studied (Fig. 5). By increasing the concentration of chitosan the total number of amino and hydroxyl groups increased, leading to more and more active sites on chitosan backbone, which anticipated that there was additional chance for MAA attacking the chitosan macro radicals that lead to an enhancement in grafting.
Effect of Polymerization Time at Different Temperatures
The impact of reaction time at different temperatures of poly(MAA)–chitosan graft copolymer is shown in Table 2. It is clear from the table that, the graft yields showing an initial fast rate, which decelerates with time, then levels off. The leveling off grafting as the reaction proceeds could be associated with reduction in monomer and initiator concentration as the reaction proceeds. While, the impact of temperature on the graft yield (%) of poly(MAA)–chitosan graft copolymer was studied by changing the reaction temperature from 45 to 75 °C, keeping other conditions fixed. As shown in Table 2, the graft yield (%) increased with increasing the temperature from 45 to 65 °C, and then decreased. The initial rise of G.Y. (%) up to 65 °C is due to the augmentation of dispersion of MAA molecules near the vicinity of the chitosan macro radicals which was helpful to improve the activity of both graft yield and homopolymerization. While, the decrement in grafting noticed after 65 °C is perhaps duo to chain transfer and termination reactions, which are well known to be favored at higher temperatures. This is in full agreement with the results published by Pengju et al. [36].
Characterization of Poly(MAA)–Chitosan Graft Copolymer as Smart Materials
Solubility in Acids and Solvents
In our trials here, we dissolved native chitosan, poly(MAA) (homopolymer) and poly(MAA)–chitosan graft copolymer (G.Y. 44.9%) in inorganic acids like (nitric and phosphorous) and organic acids like (formic, malic and acetic) in addition to common solvent like DMF, ethanol and acetone. This was done to study the suitability of the above solvents for dissolving mainly poly(MAA)–chitosan graft copolymer. Details of the conditioned used are given in the text.
The results in Table 3 disclose the following consequences:
-
1.
For all solvents used, formic, malic, acetic, phosphorus and nitric acids showed native chitosan solubility.
-
2.
Poly(MAA)–chitosan graft copolymer is insoluble in formic, acetic, and phosphorus acids in addition to DMF,
-
3.
Poly(MAA) and poly(MAA)–chitosan graft copolymer are insoluble in acetic acid and DMF respectively.
-
4.
Only nitric acid solution can be adopted as the solvent for chitosan and poly(MAA) (homopolymer) as well as poly(MAA)–chitosan graft copolymer.
In more detail, of all the eight solvents we tested, all five acids were able to dissolve native chitosan, and the ability of the acids to dissolve chitosan was greatly dependent on their pHs (0.5–2.8) as shown in Table 3. While, during the progress of grafting reaction, the solubility of poly(MAA)–chitosan graft copolymer has greatly altered in comparison with native chitosan, which was attributed to the absence of some of hydroxyl function groups in the polymers obtained with this class of redox pairs.
Water Solubility (%)
Meanwhile chitosan itself is only soluble in some specific acids or solvents as shown above; its usage has usually been limited in practical applications especially for environmental concerns [4]. Hence, the change in chitosan solubility in water is essential to successful application in industry as one of our major target in this investigation. This was done via grafting of chitosan with different concentration of soluble hydrophilic carboxyl containing monomer (MAA) using our novel redox pair initiation system. The water solubility’s (%) for poly(MAA)–chitosan graft copolymers having different graft yield (0–48%) are tabulated in Table 4. It is seen from the tabular data that, water solubility of poly(MAA)–chitosan graft copolymers increased from 0 to 20.1% by increasing the graft yield (%) from 0 to 48% in comparison of about 0% water solubility of native chitosan. This may be due to the presence of more and more hydrophilic soluble carboxyl groups on accounts of hydroxyl groups in the chitosan backbone via grafting process with this class of redox pairs as we said above in Sect. 4.1.
Super Absorbents for Water and Saline Solutions
Superabsorbent polymer is a sole material with high absorbency and good water holding characteristics. It has been applied in various fields such as health care, water-saving agriculture and materials industries. Due to the shortcomings of synthetic resins superabsorbent like poor biodegradability and high cost; superabsorbent preparation by vinyl graft copolymerization onto natural biodegradable carbohydrate polymers using hydrophilic monomers has been one of the focuses worldwide [14]. So, an absorbency test in distilled water and a saline solution (0.9% NaCl) was carried out on cross-linked poly(MAA)–chitosan graft copolymer having different graft yields ranged from (23.5–48.0) to avoid water solubility during absorption process and the result are drown in Fig. 6. The results show that the absorbency could be obtained up to 197 g H2O/g of the polymer sample and 60 g saline solution/g polymer sample for cross-linked poly(MAA)–chitosan graft copolymer having 48.0% G.Y. %. This observation is in agreement with the result reported by Chen et al. [37]. In more details, the solution absorbency of super absorbents is recognized by the concentration of the electrolytes existing in the solution i.e. carboxyl groups due to the grafting process [38]. A water absorbent system embraces a constant amount of polymer network macro radicals with different amount of aqueous ingredients. Where, the osmotic pressure related to the polymer network macro radical is the driving force for water absorbency and, subsequently, the swelling of the polymeric network macro radical. At the swelling equilibrium, the chemical potential of water in the polymer equals to the water nearby the chitosan. On the other hand, addition of sodium chloride to the cross-linked grafted chitosan solution leads to a lowering of the viscosity and the chemical potential of the water surrounding a polyelectrolyte polymer. Consequently, absorbent polyelectrolyte polymers cannot absorb as much salt water as pure water alone. In general the super absorbents are expected to be absorbing the water more than 100 times than that of their own weight. So, the results drown in Fig. 6 clearly designate that the resultant cross-linked poly(MAA)–chitosan graft copolymer prepared under the aforementioned experimental conditions fall in the category of super absorbents smart materials that; not only improves biodegradability of its corresponding superabsorbent materials, but also reduces dependence on petrochemical-derived products.
Copper Ions Removal
It is well known that, chitosan as a polycationic carbohydrate biopolymer sustains high chelating capacity for several metal ions (like Ni2+, Zn2+, Co2+, Fe2+, Mg2+ and Cu2+) in acidic conditions, and it has been frequently applied for the removal of metal ions in different industries [39]. Hence, it was essential here in our study to examine the residual and % copper ions removal of both chitosan and poly(MAA)–chitosan graft copolymer having specific graft yield (i.e. 44.9) at neutral pH for comparison. The residual copper ions were assessed over a dose of chitosan and poly(MAA)–chitosan graft copolymers concentration ranged from 0.5 to 4.0%. The obtained results are tabulated in Table 5. When the dose of chitosan and poly(MAA)–chitosan graft copolymers added to the copper solution (203 ppm Cu) was increased from 0.5 to 4.0% (w/v), the residual Cu content in the filtrate decreased gradually depending on the substrate used (Table 5). The results indicate that the residual Cu content of chitosan and poly(MAA)–chitosan graft copolymer increased with increasing concentration and levelled off at a 3.0% sample concentration. The maximal residual copper ion removal for poly(MAA)–chitosan graft copolymer and chitosan were 61 and 118 ppm (representing 73.47 and 41.87% removal) respectively, which reflect the superior copper ions removal in case of poly(MAA)–chitosan graft copolymer smart material than native chitosan. This may be attributed to the introduction of an extra functional group (i.e. carboxyl groups) onto chitosan backbone via grafting process using Mn-IV/itaconic acid initiation system. In addition to the saturation occurred at 3.0% (w/v) at which further increase in biopolymer dosage had little or no effect on copper removal. This point should be useful for launching the optimum cost-effective dose of that biopolymer in copper ion removal.
Evidence of Grafting
FT-IR of Chitosan Poly(MAA)–Chitosan Graft Copolymer
The FT-IR spectra of the chitosan and poly(MAA)–chitosan graft copolymer (44.9% G.Y.) prepared under the optimal conditions is shown in Fig. 7. It is seen from the figure that, in case of poly(MAA)–chitosan graft copolymer spectrum, the peak around 1650 cm−1 and that at 3200–3600 cm−1 in chitosan disappears and noticeable peaks at 1637 and 3450 cm−1 appear that confirms the presence of MAA on the chitosan backbone structure.
Scanning Electron Microscopy (SEM)
The surface of the graft copolymer was examined by scanning electron microscopy. The scanning electron micrographs of chitosan and poly(MAA)–chitosan graft copolymer (44.9% G.Y.) are shown in Fig. 8a, b, respectively. It is clearly seen from Fig. 8a, b that the fibrous nature of chitosan was modified after grafting. Where, the SEM image of native chitosan was smooth and no pores on the surface.
TGA of Chitosan Poly(MAA)–Chitosan Graft Copolymer
The thermal gravimetric analysis of chitosan and poly(MAA)–chitosan graft copolymer (44.9% graft yield) are given in Fig. 9. It is shown from the data that, the weight loss of native chitosan increases by increasing the temperatures up to 73.5%. While, on the other hand, in case of the weight loss of poly(MAA)–chitosan graft copolymer, the same situation was observed, but with lesser extent i.e. 66.4% (more thermal stability). Besides, the weight loss between 0 and 100 °C for both chitosan and grafted chitosan is < 10% which reflect the absorbance of water vapour by the sample. Obviously, the data drown in Fig. 9 reflect the fact that; introducing MAA moieties on the chitosan backbone via grafting using the above aforementioned redox pair increases the thermal stability of the grafted products in comparison of native chitosan. On other word, below 300 °C, poly(MAA)–chitosan graft copolymer had lower weight loss than chitosan. These mean that the grafting of chitosan surges the thermal stability of chitosan to some extent.
Conclusion
Poly(MAA)–chitosan graft copolymer was successfully prepared using our novel precipitation technique namely Mn-IV in presence of itaconic acid redox pair. The resultant copolymer was characterized by FTIR, SEM, TGA, carboxyl content and solubility % which elucidated the structure changes in comparison with native chitosan. The optimum grafting conditions were obtained at 0.1 M KMnO4, 25.9 mmol/100 g sample MnO2, 100 mmol/L itaconic acid, (50% based on weight of substrate), MAA, liquor ratio 25, 2 g chitosan, reaction time, 2 h, and reaction temperature; 65 °C. Furthermore, only concentrated nitric acid solution can be adopted as the solvent for chitosan and poly methacrylic acid (homopolymer) as well as poly(MAA)–chitosan graft copolymer. Moreover, the resultant poly(MAA)–chitosan graft copolymer as a smart material showed super absorbent hydrogel for water (197 g H2O/g polymer) and (60 g saline solution/g polymer) respectively. Besides, 73.5% copper ion removal in addition to 21% enhancement in water solubility than native chitosan. Finally, this work has the potential for promoting cost-effective and eco-friendly technologies and techniques.
References
Brine CJ, Sanford PA, Zikakis JP (eds) (1992) Advances in chitin and chitosan. Elsevier Applied Science, London
Jayakumara R, Prabaharana M, Reisa RL, Manoa JF (2005) Graft copolymerized chitosan—present status and applications. Carbohyd Polym 62:142–158
Stevens WF, Rao MS, Chandrkrachang S (eds) (1996) Chitin and chitosan. Asian Institute of Technology, Bangkok
Ehsan S, Parisa D, Ahmad AS (2016) A review on chitosan-based adsorptive membranes. Carbohydr Polym 152(5):419–432
Wen JL, Tze DC, Chia -Wen L (2009) Synthesis and characterization of lactobionic acid grafted pre-gylated chitosan and nanoparticle complex application. Polymer 50(17):4166–4174
Jiankang H, Dichen L, Yaxiong LY, Yao B, Bingheng L, andLian Q (2007) Fabrication and characterization of chitosan/gelatin porous scaffolds with predefined internal microstructures. Polymer 48(15):4578–4588
Yinsong W, Qian J, Ling RL, Qiqing Z (2007) The interaction between bovine serum albumin and the self-aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan. Polymer 48(14):4135–4142
David WJ, Samuel MH (2001) Review of vinyl graft copolymerization featuring recent advances toward controlled radical-based reactions and illustrated with chitin/chitosan trunk polymers. Chem Rev 101:3245–3273
Yang ZK, Yuan Y (2001) Studies on the synthesis and properties of hydroxyl azacrown ether-grafted chitosan. J Appl Polym Sci 82(8):1838–1843
Chen S, Wang Y (2001) Study on β-cyclodextrin grafting with chitosan and slow release of its inclusion complex with radioactive iodine. J Appl Polym Sci 82(10):2414–2421
Jung BO, Kim CH, Choi KS, Lee YM, Kim JJ (1999) Preparation of amphiphilic chitosan and their antimicrobial activities. J Appl Polym Sci 72(13):1713–1719
Kotze AF, Lueßen HL, De Leeuw BJ, De Boer AG, Verhoef JC, Junginger HE (1997) N-Trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (caco-2). Pharm Res 14(9):1197–1202
Thanou M, Verhoef JC, Junginger HE (2001) Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev 52(2):117–126
Huafei X (2011) Study on the preparation of superabsorbent composite of chitosan-g-poly (acrylic acid)/kaolin by in-situ polymerization. Int J Chem 3(3):69–74
Don TM, King CF, Chiu WY (2002) Synthesis and properties of chitosan-modified poly (vinyl acetate). J Appl Polym Sci 86(12):3057–3063
Yinghai L, Rongyue Z, Jianping Z, Weiqi Z, Shengxian L (2006) Graft copolymerization of sodium acrylate onto chitosan via redox polymerization. Iran Polym J 15(12):935–942
Ying-Chien C, Jan - Ying Y, Cheng-Fang T (2011) Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the Maillard reaction. Molecules 16:8504–8514
Jigar MJ, Vijay KS (2007) Ceric ammonium nitrate induced grafting of polyacrylamide onto carboxymethyl chitosan. Carbohydr Polym 67(3):427–435
Shantha KL, Bala U, Rao KP (1995) Tailor-made chitosans for drug delivery. Eur Polymer J 31(4):377–382
Sarhan AA, Monier M, Ayad DM, Badawy DS (2010) Evaluation of the potential of polymeric carriers based on chitosan-grafted-polyacrylonitrile in the formulation of drug delivery systems. J Appl Polym Sci 118(3):1837–1845
Pourjavadi A, Mahdavinia GR, Zohuriaan-Mehr MJ, Omidian H (2003) Modified chitosan I. Optimized cerium ammonium nitrate-induced synthesis of chitosan-graft-polyacrylonitrile. J Appl Polym Sci 88(8):2048–2054
Kurita K (1996) In: Salamone JC (ed) Polymeric materials encyclopedia, vol 2. CRC, Boca Raton, pp 1205–1208
Mitchell G, Wijnberg AC (1995) Standardization of methodology for chemical functions in starch derivatives. Part 1. Starch/Starke 47(2):46–52
Khalil MI, Mostafa KhM, Hebeish A (1993) Graft polymerization of acryl amide onto maize starch using potassium persulfate as initiator. Die Angew Makromol Chem 213(1):43–54
Mostafa KhM, Samarkandy AR, El-Sanabary AA (2011) Grafting onto carbohydrate polymer using novel potassium persulfate/tetramethylethylenediamine redox system for initiating grafting. Adv Polym Technol 30(2):138–149
Mostafa KhM, El-Sanabary AA (2013) Synthesis and characterization of novel smart flocculant based on poly (MAam)-pregelled starch graft copolymers and their degraded products. Adv Polym Technol 32(2):21339
Mostafa KhM, Samarkandy AR, El-Sanabary AA (2010) Synthesis and characterization of (poly (N-vinyl formamide)—pregelled starch graft copolymer. J Polym Res 17(6):889–900
Mostafa KhM, Morsy MS (2004) Modification of carbohydrate polymers via grafting of methacrylonitrile onto pregelled starch using potassium monopersulfate/Fe2+ redox pair. Polym Int 53(7):885–890
Mostafa KhM, El-Sanabary AA (2012) Harnessing of novel tailored modified pregelled starch derived products in sizing of cotton textiles. Adv Polym Technol 31(1):52–62
Mostafa KhM, Samarkandy AR, El-Sanabary AA (2010) Preparation of poly (DMAEM)-cross linked pregelled starch graft copolymer and its application in waste water treatments. Carbohydr Polym 86:491–498
Mostafa KhM, Samarkandy AR, El-Sanabary AA (2010) Removal of basic dyes from aqueous medium using novel poly (MAA)-cross linked pregelled starch graft copolymer. J Appl Polym Sci 118(5):2728–2735
Mostafa KhM, Samarkandy AR, El-Sanabary AA (2009) Preparation of poly (MAA)-cross linked pregelled starch graft copolymer and its application in waste water treatments. J Appl Polym Sci 112:2835–2846
Mostafa KhM, Morsy MS (2004) Tailoring a new sizing agent via structural modification of pregelled starch molecules: Part: 1: carboxymethylation and grafting. Starch/Starke 56(6):254–261
Hebeish A, Bayazeed A, El-Alfy E, Khalil MI (1988) Synthesis of vinyl polymer-starch composites to serve as size base materials. Starch-Stärke 40(5):191–196
Hebeish A, El-Alfy E, Bayazeed A (1988) Synthesis and properties of polyacrylamide-starch graft copolymers. Starch-Stärke 40(6):223–229
Pengju L, Yuezhen B, Yongqiang L, Ru C, Xuan W, Baoyan Z (2009) Studies on graft copolymerization of chitosan with acrylonitrile by the redox system. Polymer 50:5675–5680
Chen CC, Vassallo JC, Chatterjee PK (1985) In: Chatterjee PK (ed) Absorbency. Elsevier, Amsterdam, p 197
Hashem A, Afifi MA, El-Alfy EA, Hebeish A (2005) Synthesis, characterization and saponification of poly (AN)-starch composites and properties of their hydrogels. Am J Appl Sci 2(3):614–621
Aghdas H, Habibollah Y, Zahra M, Harri H (2013) Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan–MAA nanoparticles. Int J Biol Macromol 61:251–263
Funding
Funding was provided by National Institute of Standards, NIS, Egypt
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mostafa, K.M., Osman, E., Mahmoud, R.I. et al. Towards Synthesis, Characterization and Properties of Smart Material Based on Chitosan Using Mn-IV Itaconic Acid as a Novel Redox Pair. J Polym Environ 26, 3250–3261 (2018). https://doi.org/10.1007/s10924-018-1209-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1209-4