Abstract
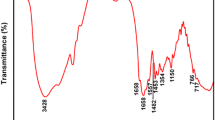
In this work, poly(methacrylatoethyl trimethyl ammonium chloride -co-acrylamide)/diatomite composite flocculant was synthesized via in situ polymerization in aqueous solution and applied in waste water treatment. The structure of composite flocculants was characterized by FT-IR, 1HNMR and XRD, TGA and viscometer. Herein, the apparent viscosity of composite flocculants was employed as comparison standard of their performance to evaluate the influence of the reaction parameters, such as monomer feeding ratio, diatomite mass fraction and polymerization temperature, etc. on their flocculation performance. And based on the above investigations, the optimum synthesis condition could be found. By comparing flocculation properties of composite flocculants with that of the conventional cationic flocculant, the dosage of composite flocculant that could make the transmittance of treated waste water exceed 95 % was only 7.5 ppm which was far lower than that of conventional flocculant (60–90 ppm). Meanwhile, the settling time was lower than 5 s which was similarly to that of conventional flocculant. Finally, the conclusion was that the composite flocculant owned higher absorption capacity and larger chain extending space than those of conventional linear flocculant due to the introduction of diatomite as backbone, which could make linear polymer chains free from entanglement and improve the flocculation capacity notably.










Similar content being viewed by others
References
Ponou J, Ide T, Suzuki A, Tsuji H, Wang LP, Dodbiba G, Fujita T (2014) Water Sci Technol 69:1249–1258
Tripathy T (2006) Vidyasagar University. Midnapore, West-Bengal
Ríos HE, González-Navarrete J, Vargas V, Urzúa MD (2011) Colloid Surf A 384:262–267
Borkovec M, Papastavrou G (2008) Curr Opin Colloid Interface Sci 13:429–437
Granados MR, Acién FG, Gómez C, Fernández-Sevilla JM, Grima EM (2012) Bioresour Technol 118:102–110
Chen X, Chen G, Yue PL (2007) Sep Sci Technol 19:65–76
Wang JP, Chen YZ, Ge XW, Yu HQ (2007) Chemosphere 66:1752–1757
Wang LJ, Wang JP, Yuan SJ, Zhang SJ, Yong T, Yu HQ (2009) Chem Eng J 149:118–122
Ho YC, Norli I, Alkarkhi AFM, Morad N (2010) Bioresour Technol 101:1166–1174
Gao BY, Wang Y, Yue QY (2005) Acta Hydrochim Hydrobiol 33:365–371
Zou J, Zhu H, Wang F, Sui H, Fan J (2011) Chem Eng J 171:350–356
Lemons JF (1997) Am Ceram Soc Bull 76:92–98
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) Water Res 33:2469–2479
Al-Degs Y, Khraisheh MAM, Tutunji MF (2001) Water Res 35:3724–3728
Karaman S, Karaipekli A, Sarı A, Biçer A (2011) Sol Energy Mater Sol Cells 95:1647–1653
Xu B, Li Z (2013) Appl Energy 105:229–237
Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) J Environ Manag 69:229–238
Al-Ghouti MA, Al-Degs YS (2011) Chem Eng J 173:115–128
O’Shea JP, Qiao GG, Franks GV (2010) J Colloid Interface Sci 348:9–23
Sun Z, Zhang Y, Zheng S, Park Y, Frost RL (2013) Thermochim Acta 558:16–21
Shen WN, Feng LJ, Feng H (2012) Chem J Chin Univ 33:353–360
Barata-Rodrigues PM, Mays TJ, Moggridge GD (2003) Carbon 41:2231–2246
Ovenden C, Xiao H (2002) Colloid Surf A 197:225–234
Ji J, Qiu J, Wai N, Wong FS, Li Y (2009) Water Res 44:1627–1635
Biggs S, Habgood M, Jameson GJ, Yan YD (2000) Chem Eng J 80:13–22
Gregory J, Barany S (2011) Adv Colloid Interface Sci 169:1–12
Gao BY, Yan W, Yue QY, Wei JC, Qian L (2007) Sep Purif Technol 54:157–163
Bolto B, Gregory J (2007) Water Res 41:2301–2324
Wei J, Gao B, Yue Q, Wang Y, Li W, Zhu X (2009) Water Res 43:724–732
Acknowledgments
The authors are grateful for the financial support provided by Major Project of Jilin Province (Nos. 20140204083GX, 20160101306JC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Xu, K., Liu, Y., Wang, Y. et al. A Novel Wastewater Treating Material: Cationic Poly Acrylamide/Diatomite Composite Flocculant. J Polym Environ 26, 3051–3059 (2018). https://doi.org/10.1007/s10924-018-1176-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1176-9