Abstract
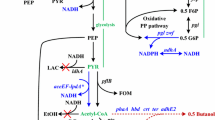
The high production cost of bio-based plastic polyhydroxyalkanoates (PHAs) limits their use as commercial products. Thus, systems for PHAs production from waste substrates could reduce production costs. Crude glycerol is a by-product of biodiesel fuel production and thus represents an inexpensive, abundant and promising carbon source for production of valorized fermentation products. In this study, industrial crude glycerol by-product from palm oil biodiesel production was used as the carbon source for production of PHAs by recombinant Escherichia coli. Crude glycerol supplemented at 1–5 % (v/v) supported production of poly(3-hydroxybutyrate) (P(3HB)) in E. coli-ABC Ah , which harbors the PHA synthetic genes for β-ketothiolase (PhaA Re ), acetoacetyl-CoA reductase (PhaB Re ) of Ralstonia eutropha and Polyhydroxyalkanoate (PHA) synthase (PhaC Ah ) of Aeromonas hydrophila. The highest P(3HB) content and productivity of 14 wt% of cell dry weight and 0.6 g/L, respectively, were obtained at 1 % (v/v) glycerol concentration. Production of P(3HB-co-3-hydroxyhexanoate) (P(3HB-co-3HHx)) was achieved using E. coli-ABC Ah J Ah , harboring genes for PhaA Re , PhaB Re , PhaC Ah , and the (R)-specific enoyl-CoA hydratase (PhaJ Ah ) of A. hydrophila. This led to the copolymer content of 3 wt% of cell dry weight with 1 mol% of 3HHx. Molecular weight and degradation temperature of the polymers were in the range of 110–130 kDa and 295–299 °C, respectively. These results indicated that crude glycerol could be an attractive carbon source for economical production of PHAs with properties for industrial application.



Similar content being viewed by others
References
Yang F, Hanna MA, Sun R (2012) Biotechnol Biofuels 5:13
da Silva GP, Mack M, Contiero J (2009) Biotechnol Adv 27:30–39
Peters D (2007) Adv Biochem Eng Biotechnol 105:1–30
Pyle DJ, Garcia RA, Wen Z (2008) J Agric Food Chem 56:3933–3939
Ito T, Nakashimada Y, Senba K, Matsui T, Nishio N (2005) J Biosci Bioeng 100:260–265
Mattam AJ, Clomburg JM, Gonzalez R, Yazdani SS (2013) Biotechnol Lett 35:831–842
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) J Appl Microbiol 102:1437–1449
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Appl Microbiol Biotechnol 57:50–55
Budde CF, Riedel SL, Willis LB, Rha C, Sinskey AJ (2011) Appl Environ Microb 77:2847–2854
Mothes G, Schnorpfeil C, Ackermann J-U (2007) Eng Life Sci 7:475–479
Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR (2009) Process Biochem 44:509–515
Ashby RD, Solaiman DKY, Foglia AT (2004) J Polym Environ 12:105–112
Phithakrotchanakoon C, Champreda V, Aiba S, Pootanakit K, Tanapongpipat S (2013) Biosci Biotechnol Biochem 77:1262–1268
Tsuge T, Taguchi K, Taguchi S, Doi Y (2003) Int J Biol Macromol 31:195–205
Watanabe Y, Ichinomiya Y, Shimada D, Saika A, Abe H, Taguchi S, Tsuge T (2012) J Biosci Bioeng 113:286–292
Lee SJ, Kim SB, Kang SW, Han SO, Park C, Kim SW (2012) Bioproc Biosyst Eng 35:85–92
Desbois AP, Smith VJ (2010) Appl Microbiol Biotechnol 85:1629–1642
Shimamura E, Kasuya K, Kobayashi G, Shiotani T, Shima Y, Doi Y (1994) Macromolecules 27:878–880
Kawata Y, Aiba S (2010) Biosci Biotechnol Biochem 74:175–177
Asrar J, Valentin HE, Berger PA, Tran M, Padgette SR, Garbow JR (2002) Biomacromolecules 3:1006–1012
Zhu C, Nomura CT, Perrotta JA, Stipanovic AJ, Nakas JP (2010) Biotechnol Progr 26:424–430
Madden LA, Anderson AJ, Shah DT, Asrar J (1999) Int J Biol Macromol 25:43–53
Foster LJR (2010) Biopolymers 243–256
Acknowledgments
We would like to thank Dr. Isao Noda for kindly providing standard P(3HB-co-3HHx) (Nodax™). Manuscript proofreading by Dr. Philip Shaw is appreciated. This work was supported by the Royal Golden Jubilee Ph.D. Program Scholarship Program (PHD/0268/2549), Mahidol University and a research grant from the National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phithakrotchanakoon, C., Champreda, V., Aiba, Si. et al. Production of Polyhydroxyalkanoates from Crude Glycerol Using Recombinant Escherichia coli . J Polym Environ 23, 38–44 (2015). https://doi.org/10.1007/s10924-014-0681-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-014-0681-8