Abstract
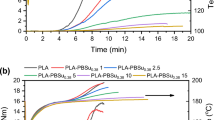
Solid state polymerizations (SSP) and the kinetic behavior in melt state of l-lactide polymerizations employing magnesium stearate as catalyst were investigated. The solid state polymerizations were carried out in two steps where pre-polymers were first prepared in melt polymerizations at 180 °C and the subsequent post-polymerizations were performed around the Tc of polylactide (PLA). In order to find the initial SSP conditions, kinetic profiles of melt polymerizations of l-lactide with magnesium stearate were determined. According to the kinetics data the melt polymerizations were found to be first order with respect to lactide as evident from a linear relationship of logarithmic variations of l-lactide concentration versus time using catalyst/monomer ratios of 1:500 and 1:5,000. When the catalyst content is increased to 1:100 the relationship loses its linearity due to fast propagation in the early stages of the reaction. From the GPC data it can be noted that the molecular weight of PLA can be increased by 5–17 times under the conditions established for our SSP experiments. A comparison between the two step solid state polymerizations and already reported melt polymerizations using the same catalyst showed that SSP furnished polymers with much lower amount of polymer degradation.




Similar content being viewed by others
References
Gironi F, Piemonte G (2011) Environ Prog Sustain Energy 30(3):459–468
Patel M, Bastioli C, Marini L, Würdinger E (2005) Biopolymers Online ed. Wiley-VCH Verlag GmbH & Co
Laine P, Kontio R, Lindqvist C, Suuronen R (2004) Int J Oral Maxillofac Surg 33:240–244
Liang LS, Wong W, Burt HM (2005) J Pharm Sci 94:1204–1215
Schmidmaier G, Wildemann B, Stemberger A, Haas NP, Raschke M (2001) J Biomed Mater Res 58:449–455
Yu Y, Storti G, Morbidelli M (2011) Ind Eng Chem Res 50:7927–7940
Kricheldorf HR, Hachmann-Thiessen H, Schwarz G (2004) Biomacromolecules 5:492–496
Dechy-Cabaret O, Martin-Vaca B, Bourissou D (2004) Chem Rev 104(12):6147–6176
Wheaton CA, Hayes PG, Ireland BJ (2009) Dalton Trans 25:4832–4846
Dobrzynski P, Kasperczyk J, Janeczek H, Bero M (2002) Polymer 43:2595–2601
Dias ML, Palermo LC, Silvino AC (2011) Macromol Symp 299–300:156–163
Kricheldorf HR, Serra A (1985) Polym Bull 14:497–502
FDA’s SCOGS Database (1979) Report No. 60; ID Code: 557-04-0
Sworbrick J, Boylan JC (1995) Encyclopedia of pharmaceutical technology, vol 12. Marcel Dekker, New York, pp 81–103
Katiyar V, Shaama MS, Nanavati H (2011) J Appl Polym Sci 122:2966–2980
Hermans PH, Weidinger A (1961) Macromol Chem 44:24–36
Savitzky A, Golay MJE (1964) Anal Chem 36(8):1627–1639
Wojdyr MJ (2010) Appl Cryst 43:1126
Shyamroy S, Garnaik B, Sivaran S (2005) J Polym Sci Polym Chem 43:2164
Chrisholm MH, Eilerts NW, Huffman JC, Iyer SS, Pacold M, Phomphrai K (2000) J Am Chem Soc 122:11845
Stridsberg KM, Ryner M, Albertsson A-C (2002) Adv Polym Sci 157:42–65
Zhong Z, Schneiderbauer S, Dijkstra PJ, Westerhausen M, Feijen J (2002) J Polym Environ 9:31–38
Nijenhuis AJ, Grijpma DW, Pennings AJ (1992) Macromolecules 25:6414
Katiyar V, Nanavati H (2011) Polym Eng Sci 51(10):2078–2084
Shinno K, Miyamoto M, Kimura Y (1997) Macromolecules 30:6438–6444
Auras R, Lim L-T, Selke SEM, Tsuji H (2010) Poly(lactic acid): synthesis, structures, properties, processing, and applications. Wiley, New Jersey
Degée P, Dubois P, Jérôme R (1997) Macromol Chem Phys 198:1985–1995
Fan Y, Nishida H, Shirai Y, Endo T (2003) Polym Degrad Stab 80:503–511
Noda M, Okuyama H (1999) Chem Pharm Bull 47:467–471
Södergards A, Näsman JH (1996) Ind Eng Chem Res 35:732–735
Kowalski A, Duda A, Penczek S (2000) Macromolecules 33:7359–7370
Acknowledgments
The authors are grateful to the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silvino, A.C., de Abreu Talina Martins, D.B., da Costa Rodrigues, A. et al. Kinetic Behavior in Melt State and Solid State Polymerization of Lactide Using Magnesium Stearate as Catalyst. J Polym Environ 21, 1002–1008 (2013). https://doi.org/10.1007/s10924-013-0603-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-013-0603-1