Abstract
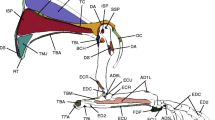
Tree sloths have reduced skeletal muscle mass, and yet they are able to perform suspensory behaviors that require both strength and fatigue resistance to suspend their body mass for extended periods of time. The muscle architecture of sloths is hypothesized to be modified in ways that will enhance force production to compensate for this reduction in limb muscle mass. Our objective is to test this hypothesis by quantifying architecture properties in the forelimb musculature of the brown-throated three-toed sloth (Bradypus variegatus: N = 4). We evaluated architecture from 52 forelimb muscles by measuring muscle moment arm (r m), muscle mass (MM), belly length (ML), fascicle length (LF), pennation angle (θ), and physiological cross-sectional area (PCSA), and these metrics were used to estimate isometric force, joint torque, and power. Overall, the musculature becomes progressively more pennate from the extrinsic to intrinsic regions of the forelimb, and the flexors are more well developed than the extensors as predicted. However, most muscles are indicative of a mechanical design for fast joint rotational velocity instead of large joint torque (i.e., strength), although certain large, parallel-fibered shoulder (e.g., m. latissimus dorsi) and elbow (e.g., m. brachioradialis) flexors are capable of producing appreciable torques by having elongated moment arms. This type of functional tradeoff between joint rotational velocity and mechanical advantage is further exemplified by muscle gearing in Bradypus that pairs synergistic muscles with opposing LF/r m ratios in each functional group. These properties are suggested to facilitate the slow, controlled movements in sloths. In addition, the carpal/digital flexors have variable architectural properties, but their collective PCSA and joint torque indicates the capability for maintaining grip force and carpal stability while distributing load from the manus to the shoulder. The observed specializations provide a basis for understanding sustained suspension in sloths.







Similar content being viewed by others
References
Alexander R, Jayes AS, Maloiy GMO, Wathuta EM (1981) Allometry of the leg muscles of mammals. J Zool 194:539–552
Biewener AA (2005) Biomechanical consequences of scaling. J Exp Biol 208:1665–1676
Britton SW, Kline RF (1939) Augmentation of activity in the sloth by adrenal extract, emotion and other conditions. Am J Physiol 127:127–130
Channon AJ, Günther MM, Crompton RH, Vereecke EE (2009) Mechanical constraints on the functional morphology of the gibbon hind limb. J Anat 215:383–400
Cliffe RN, Haupt RJ, Avey-Arroyo JA, Wilson RP (2015) Sloths like it hot: ambient temperature modulates food intake in the brown-throated sloth (Bradypus variegatus). Peer J 3:875–889
De Moura Filho AG, Huggins SE, Lines SG (1983) Sleep and waking in the three-toed sloth, Bradypus tridactylus. Comp Biochem Physiol A Comp Physiol 76: 345–355
Elissamburu A, De Santis L (2011) Forelimb proportions and fossorial adaptations in the scratch-digging rodent Ctenomys (Caviomorpha). J Mammal 92:683–689
Emerling CA, Springer MS (2015) Genomic evidence for rod monochromacy in sloths and armadillos suggests early subterranean history for Xenarthra. Proc R Soc B 282:20142192
Engelmann GF (1985) The phylogeny of the Xenarthra. In: Montgomery GG (ed) The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas. Smithsonian Institution Press, Washington, pp 51–64
Ettema GJC, Styles G, Kippers V (1998) The moment arms of 23 muscle segments of the upper limb with varying elbow and forearm positions: implications for motor control. Hum Mov Sci 17:201–220
Garner BA, Pandy MG (2003) Estimation of musculotendon properties in the human upper limb. Ann Biomed Eng 31:207–220
Genoways HH, Timm RM (2003) The xenarthrans of Nicaragua. J Neotropical Mammal 10:231–253
Granatosky MC, Schmitt D (2017) Forelimb and hind limb loading patterns during below branch quadrupedal locomotion in the two-toed sloth. J Zool 302:271–278
Grand TI (1978) Adaptations of tissue and limb segments to facilitate moving and feeding in arboreal folivores. In: Montgomery GG (ed) The Ecology of Arboreal Folivores. Smithsonian Institution Press, Washington, D.C., pp 231–241
Hayssen V (2009) Bradypus tridactylus (Pilosa: Bradypodidae). Mammal Species 839:1–9
Hayssen V (2010) Bradypus variegatus (Pilosa: Bradypodidae). Mammal Species 42:19–32
Hayssen V (2011) Choloepus hoffmanni (Pilosa: Megalonychidae). Mammal Species 43:37–55
Hildebrand M (1960) How animals run. Sci Am 202:148–157
Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc B 126:136–195
Hudson PE, Corr SA, Payne-Davis RC, Clancy SN, Lane E, Wilson AM (2011) Functional anatomy of the cheetah (Acinonyx jubatus) forelimb. J Anat 218:375–385
Humphrey GM (1869) Myology of the limbs of the unau, ai, two-toed anteater, and pangolin. J Anat Phys 4:17–78
International Committee on Veterinary Gross Anatomical Nomenclature (2012) Nomina Anatomica Veterinaria, 5th edn. Editorial Committee, Hannover
Lieber RL (2009) Skeletal Muscle, Structure, Function, and Plasticity, 3rd edition. Lippincott Williams & Wilkins Baltimore, 369 pp
Lieber RL, Blevins FT (1989) Skeletal muscle architecture of the rabbit hindlimb: functional implications of muscle design. J Morphol 199:93–101
Macalister A (1869) On the myology of Bradypus tridactylus; with remarks on the general muscular anatomy of the Edentata. Trans R Irish Acad 25:51–67
McClearn D (1985) Anatomy of raccoon (Procyon lotor) and coati (Nasua narica and N. nasua) forearm and leg muscles: relations between fiber length, moment-arm length, and joint-angle excursion. J Morphol 183:87–115
McDonald HG, De Iuliis G (2008) Fossil history of sloths. In: Vizcaíno SF, Loughry WJ (eds) The Biology of the Xenarthra. University Press of Florida, Gainesville, pp 39–55
Medler S (2002) Comparative trends in shortening velocity and force production in skeletal muscles. Am J Physiol 283:R368-R378
Mendel FC (1981a) Use of hands and feet of two-toed sloths (Choloepus hoffmanni) during climbing and terrestrial locomotion. J Mammal 62:413–421
Mendel FC (1981b) The hand of two-toed sloths (Choloepus): Its anatomy and potential uses relative to size of support. J Morphol 169:1–19
Mendel FC (1985a) Use of hands and feet of three-toed sloths (Bradypus variegatus) during climbing and terrestrial locomotion. J Mammal 66:359–366
Mendel FC (1985b) Adaptations for suspensory behavior in the limbs of two-toed sloths In: Montgomery GG (ed) The Ecology and Evolution of Armadillos, Sloths, and Vermilinguas. Smithsonian Institute Press, Washington, D.C., pp 151–162
Mendez J, Keyes A (1960) Density and composition of mammalian muscle. Metabolism 9:184–188
Miller RA (1935) Functional adaptations in the forelimb of the sloths. J Mammal 16:38–51
Moore AL, Budny JE, Russell AP, Butcher MT (2013) Architectural specialization of the intrinsic forelimb musculature of the American badger (Taxidea taxus). J Morphol 274:35–48
Muchlinski MN, Snodgrass JJ, Terranova CJ (2012) Muscle mass scaling in primates: an energetic and ecological perspective. Am J Primatol 74:395–407
Nyakatura JA (2012) The convergent evolution of suspensory posture and locomotion in tree sloths. J Mammal Evol 19:225–234
Nyakatura JA, Andrada E (2013) A mechanical link model of two-toed sloths: no pendular mechanics during suspensory locomotion. Acta Theriol 58:83–93.
Nyakatura JA, Fischer MS (2011) Functional morphology of the muscular sling at the pectoral girdle in tree sloths: convergent morphological solutions to new functional demands? J Anat 219:360–374
Olson RA, Womble MD, Thomas DR, Glenn ZD, Butcher MT (2016) Functional morphology of the forelimb of the nine-banded armadillo (Dasypus novemcinctus): comparative perspectives on the myology of Dasypodidae. J Mammal Evol 23:49–69
Pate E, Wilson GJ, Bhimani M, Cooke R (1994) Temperature dependence of the inhibitory effects of orthovanadate on shortening velocity in fast skeletal muscle. Biophys J 66:1554–1562
Pauli JN, Peery MZ, Fountain ED, Karasov WH (2016) Arboreal folivores limit their energetic output, all the way to slothfulness. Am Nat 188:196–204
Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM (2005) Functional specialisation of pelvic limb anatomy in horses (Equus caballus). J Anat 206:557–574
Preuschoft H, Demes B (1984) Biomechanics in brachiation. In: Preuschoft H, Chivers DJ, Brockelman WY, Creel N (eds) The Lesser Apes: Evolutionary and Behavioral Biology. Edinburgh University Press, Edinburgh, pp 96–118
Ranatunga KW (1996) Endothermic force generation in fast and slow mammalian (rabbit) muscle fibers. Biophys J 71:1905–1913
Rose JA, Moore AL, Russell AP, Butcher MT (2014) Functional osteology of the forelimb digging apparatus in badgers. J Mammal 95:543–558
Rose JA, Sandefur A, Huskey S, Demler JL, Butcher MT (2013) Muscle architecture and out-force potential of the thoracic limb in the eastern mole (Scalopus aquaticus). J Morphol 274:1277–1287
Rupert JE, Rose JA, Organ JM, Butcher MT (2015) Forelimb muscle architecture and myosin isoform composition in the groundhog (Marmota monax). J Exp Biol 218:194–205
Sacks RD, Roy RR (1982) Architecture of the hind limb muscles of cats: functional significance. J Morphol 173:185–195
Schinz HR (1825) Das Thierreich eingetheilt nach dem Bau der Thiere als Grundlage ihrer Naturgeschichte und der vergleichenden Anatomie. Vol. 4. J. G. Cotta, Stuttgart
Spainhower K, Metz AK, Kiraly PM, Thomas DR, Butcher MT (2017) Fiber type properties of the limb muscles of sloths (Xenarthra: Pilosa). Integr Comp Biol 57(suppl 1): e414, 227
Stalheim-Smith A (1989) Comparison of the muscle mechanics of the forelimb of three climbers. J Morphol 202:89–98
Toniolo L, Maccatrozzo L, Patruno M, Pavan E, Caliaro F, Rossi R, Rinaldi C, Canepari M, Reggiani C, Mascarello F (2007) Fiber types in canine muscles: myosin isoform expression and functional characterization. Am J Physiol 292:C1915-C1926
Vizcaíno SF, Milne N (2002) Structure and function in armadillo limbs (Mammalia: Xenarthra: Dasypodidae). J Zool 257:117–127
Warburton NM, Grégoire L, Jacques S, Flandrin C (2013) Adaptations for digging in the forelimb muscle anatomy of the southern brown bandicoot (Isoodon obesulus) and bilby (Macrotis lagotis). Aust J Zool 61:402–419
Williams SB, Payne RC, Wilson AM (2007a) Functional specialisation of the pelvic limb of the hare (Lepus europeus). J Anat 210:472–490
Williams SB, Payne RC, Wilson AM (2007b) Functional specialisation of the thoracic limb of the hare (Lepus europeus). J Anat 210:491–505
Williams SB, Wilson AM, Daynes J, Peckham K, Payne RC (2008a) Functional anatomy and muscle moment arms of the thoracic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris). J Anat 213:373–382
Williams SB, Wilson AM, Rhodes L, Andrews J, Payne RC (2008b) Functional anatomy and muscle moment arms of the pelvic limb of an elite sprinting athlete: the racing greyhound (Canis familiaris). J Anat 213:361–372
Woledge RC, Curtin NA, Homsher E (1985) Energetic aspects of muscle contraction. Monogr Physiol Soc 41:1–357
Acknowledgments
We sincerely thank Judy Avey-Arroyo and Gerald Richardson for their research partnership with The Sloth Sanctuary of Costa Rica and access to frozen sloth specimens. We also thank the entire staff at The Sloth Sanctuary for making us very comfortable during our stay. A very special thanks to Regan Falin for the anatomical illustrations. Thanks to Sarah Kennedy and Dylan Thomas for assistance with data collection and photography. Support by the American Society of Mammalogists (ASM) Grants-in-Aid of Research (GIAR) funding to RA Olson. The YSU College of STEM also provided travel funding to ZD Glenn (current address: Ohio University College of Osteopathic Medicine). The YSU Department of Biological Sciences, Ohio University Department of Biological Sciences, and Swansea University Department of Biosciences are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplemental Fig 1
Mean summed physiological cross-sectional area (PCSA) distribution across selected functional muscle groups in the forelimb of B. variegatus. Data shown are only for adult individuals. The functional muscle groups are bracketed by their actions at each major limb joint and include the total summed PCSA (in cm2) for each subgroup. Bars are means ± SD (N = 3). (GIF 71 kb)
ESM 1
(DOCX 47 kb)
Rights and permissions
About this article
Cite this article
Olson, R.A., Glenn, Z.D., Cliffe, R.N. et al. Architectural Properties of Sloth Forelimb Muscles (Pilosa: Bradypodidae). J Mammal Evol 25, 573–588 (2018). https://doi.org/10.1007/s10914-017-9411-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9411-z