Abstract
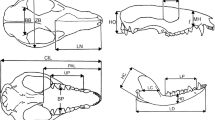
The patterns of development and skull ontogeny in caenolestids have been poorly studied, resulting in a limited knowledge. In this work, we report and compare the allometric growth trends of 15 variables in the three living groups of the Family Caenolestidae, represented by Caenolestes fuliginosus, Lestoros inca, and Rhyncholestes raphanurus. We analyzed the bivariate and multivariate allometry in comparison with morphologically convergent Australasian peramelids, as well as with other marsupials and placentals previously studied. We also report the phylogenetic signal and optimization of the confidence intervals of the variables analyzed in two alternative hypotheses, where Ameridelphia is considered as monophyletic and paraphyletic. Rhyncholestes raphanurus and C. fuliginosus shared more allometric trends than any other between-taxa comparisons. Notwithstanding, several statistics were higher in R. raphanurus, except for those variables related to temporal muscles and bite. The close relationship between R. raphanurus and L. inca is also supported by the longitudinal growth of the rostrum, although with a clear growth extension in R. raphanurus. The allometric trends reported for L. inca reflect a more predaceous condition compared to other caenolestids. Bandicoots and caenolestids did not show a particularly shared growth pattern, with the latter being morphologically more conservative. Ameridelphia was paraphyletic in the shortest tree regarding the optimization of the confidence intervals. However, the growth of several variables supported monophyletic groups in both hypotheses. Skull ontogeny in marsupials is informative in several aspects of the mandible and neurocranium reflecting the high phylogenetic signal displayed by variables related to these cranial regions.




Similar content being viewed by others
References
Abdala F, Flores DA, Giannini N (2001) Postweaning ontogeny of the skull of Didelphis albiventris. J Mammal 82:190–200. doi:10.1644/1545-1542(2001)082<0190:POOTSO>2.0.CO;2
Abello MA (2007). Sistemática y bioestratigrafía de los Paucituberculata (Mammalia, Marsupialia) del Cenozoico de América del Sur. Dissertation, Universidad Nacional de La Plata, Argentina
Abello MA (2013) Analysis of dental homologies and phylogeny of Paucituberculata (Mammalia: Marsupialia). Biol J Linn Soc 109:441–465. doi:10.1111/bij.12048
Amrine-Madsen H, Scally M, Westerman M, Stanhope MJ, Krajewski C, Springer MS (2003) Nuclear gene sequences provide evidence for the monophyly of australidelphian marsupials. Mol Phylogen Evol 28:186–196. doi:10.1016/S1055-7903(03)00122-2
Archer M (1976) The basicranial region of marsupicarnivores (Marsupialia), inter-relationships of carnivorous marsupials, and affinities of the insectivorous marsupial peramelids. Zool J Linn Soc 59:217–322
Asher R, Horovitz I, Sánchez-Villagra M (2004) First combined cladistic analysis of marsupial mammal interrelationships. Mol Phylogen Evol 33:240–250. doi:10.1016/j.ympev.2004.05.004
Astúa D (2010) Cranial sexual dimorphism in New World marsupials and a test of Rensch’s rule in Didelphidae. J Mammal 91:1011–1024. doi:10.1644/09-MAMM-A-018.1
Beck RMD (2008) A dated phylogeny of marsupials using a molecular supermatrix and multiple fossil constraints. J Mammal 89:175–189. doi:10.1644/06-MAMM-A-437.1
Beck RMD, Godthelp H, Weisbecker V, Archer M, Hand SJ (2008) Australia’s oldest marsupial fossils and their biogeographical implications. PLoS ONE. doi:e1858. doi:10.1371/journal.pone.0001858
Bennett CV, Goswami A (2013) Statistical support for the hypothesis of developmental constraint in marsupial skull evolution. BMC Biology 11. doi:10.1186/1741-7007-11-52
Biggers JD, Delamater ED (1965) Marsupial spermatozoa pairing in the epididymis of American forms. Nature 208:402–404
Birney EC, Sikes RS, Monjeau JA, Guthmann N, Phillips CJ (1996) Comments on Patagonian marsupials from Argentina. In: Genoways HH, Baker RJ (eds) Contributions in Mammalogy: A Memorial Volume Honoring Dr. J. Knox Jones, Jr. Museum of Texas Tech University, pp 149–154
Brown BE (2004) Atlas of New World marsupials. Fieldiana Zool 102:1–308
Bruniard ED (1982) La diagonal árida argentina: un límite climático real. Rev Geog 95:5–20
Bublitz J (1987) Untersuchungen zur Systematik der Rezenten Caenolestidae Trouessart, 1898: Unter Verwendung craniometrischer Methoden. Bonn Zool Monog 23:1–96
Burbidge A, Dickman C, Johnson K (2008) Chaeropus ecaudatus. IUCN Red List of Threatened Species. Version 2013.2. http://www.iucnredlist.org. Accessed 25 April 2014
Cabrera A (1957) Catálogo de los Mamíferos de América del Sur. Rev Mus Arg Cs Nat “Bernardino Rivadavia” 4:1–308
Cardillo M, Bininda-Emonds O, Boakes E, Purvis A (2004) A species-level phylogenetic supertree of marsupials. J Zool 264:11–31. doi:10.1017/S0952836904005539
Chimento N, Agnolin F, Novas F (2012) The Patagonian fossil mammal Necrolestes: a Neogene survivor of Dryolestoidea. Rev Mus Arg Cs Nat “Bernardino Rivadavia” 14:261–306
Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215:119–149. doi:10.1002/jmor.1052150203
Colgan DJ (1999) Phylogenetic studies of marsupials based on phosphoglycerate kinase DNA sequences. Mol Phylogen Evol 11:13–26. doi:10.1006/mpev.1998.0553
Emerson SB, Bramble DM (1993) Scaling, allometry and skull design. In: Hanken J, Hall BK (eds) The Skull, Volume 3. Functional and Evolutionary Mechanisms. The University of Chicago Press, Chicago, pp 384–416
Farris J (1970) Methods for computing Wagner trees. Syst Zool 19:83–92. doi:10.1093/sysbio/19.1.83
Flores DA, Casinos A (2006) Cranial ontogeny and sexual dimorphism in two New World monkeys: Alouatta caraya (Atelidae) and Cebus apella (Cebidae). J Morphol 272:744–757. doi:10.1002/jmor.10947
Flores DA, Giannini N, Abdala F (2003) Cranial ontogeny of Lutreolina crassicaudata (Didelphidae): a comparison with Didelphis albiventris. Acta Theriol 48:1–9. doi:10.1007/BF03194261
Flores DA, Giannini N, Abdala F (2006) Comparative postnatal ontogeny of the skull in the australidelphian metatherian Dasyurus albopunctatus (Marsupialia: Dasyuromorpha: Dasyuridae). J Morphol 267:426–440. doi:10.1002/jmor.10420
Flores DA, Abdala F, Giannini N (2010) Cranial ontogeny of Caluromys philander (Didelphidae, Caluromyinae): a qualitative and quantitative approach. J Mammal 91:539–550. doi:10.1644/09-MAMM-A-291.1
Flores DA, Abdala F, Giannini N (2013) Post-weaning cranial ontogeny in two bandicoots (Mammalia, Peramelomorphia, Peramelidae) and comparison with carnivorous marsupials. Zoology 116:372–384. doi:10.1016/j.zool.2013.07.003
Freedman L, Joffe AD (1967) Skull and tooth variation in the genus Perameles, Part 2: metrical features of P. nasuta. Rec Austral Mus 27:183–195
Freedman L, Rightmire GP (1971) Skull and tooth variation in Australian bandicoots (Peramelidae, Marsupialia): the genus Isoodon and multivariate comparisons with Perameles. J Roy Soc West Austral 54:21–31
Gardner A (2005) Order Paucituberculata. In: Wilson DE, Reeder DM (eds) Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd ed. Johns Hopkins University Press, Baltimore, pp 19–20
Gemmell NJ, Westerman M (1994) Phylogenetic relationships within the Class Mammalia: a study using mitochondrial 12S RNA sequences. J Mammal Evol 2:3–23. doi:10.1007/BF01464347
Giannini N (2014) Quantitative developmental data in a phylogenetic Framework. J Exp Zool doi:10.1002/jez.b.22588
Giannini NP, Goloboff P (2010) Delayed-response phylogenetic correlation, an optimization based method to test covariation of continuous characters. Evolution 64:1885–1898. doi:10.1111/j.1558-5646.2010.00956.x
Giannini NP, Abdala F, Flores DA (2004) Comparative postnatal ontogeny of the skull in Dromiciops gliroides (Marsupialia: Microbiotheriidae). Am Mus Novitates 3460:1–17. http://hdl.handle.net/2246/2770
Giannini NP, Segura V, Giannini MI, Flores D (2010) A quantitative approach to the cranial ontogeny of the Puma. Mammal Biol 75:547–554. doi:10.1016/j.mambio.2009.08.001
Goin FJ, Candela AM, Muizon C de (2003) The affinities of Roberthoffstetteria nationalgeographica (Marsupialia) and the origin of the polydolopine molar pattern. J Vertebr Paleontol 23:869–869. doi:10.1671/2383-11
Goin FJ, Sánchez-Villagra MR, Abello A, Kay RF (2007) A new generalized paucituberculatan marsupial from the Oligocene of Bolivia and the origin of ‘shrew-like’ opossums. Palaeontology 50: 1267–1276. doi:10.1111/j.1475-4983.2007.00706.x
Goin FJ, Candela AM, Abello A, Oliveira EV (2009) Earliest South American paucituberculatans and their significance in the understanding of ‘pseudodiprotodont’ marsupial radiations. Zool J Linn Soc 155:867–884. doi:10.1111/j.1096-3642.2008.00471.x
Goloboff PA, Mattoni CI, Quinteros AS (2006) Continuous characters analyzed as such. Cladistics 22:589–601. doi:10.1111/j.1096-0031.2006.00122.x
Goloboff PA, Farris JS, Nixon KC (2008) TNT, a free program for phylogenetic analysis. Cladistics 24:774–786. doi:10.1111/j.1096-0031.2008.00217.x
Gordon G, Hulbert AJ (1989) Peramelidae. In: Walton DW, Richardson DJ (eds) Fauna of Australia. Australian Government Publishing Service, Sydney, pp 1–42
Goswami A, Milne N, Wroe S (2011) Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc Roy Soc B 278:1831–1839. doi:10.1098/rspb.2010.2031
Goswami A, Polly PD, Mock OB, Sánchez Villagra MR (2012) Shape, variance and integration during craniogenesis: contrasting marsupial and placental mammals. J Evol Biol 25:862–872. doi:10.1111/j.1420-9101.2012.02477.x
Gregory WK (1922) On the “habitus” and “heritage” of Caenolestes. J Mammal 3:106–14. doi:10.2307/1373300
Groves CP (2005) Order Peramelemorphia. In: Wilson DE, Reeder DB (eds) Mammal Species of the World: A Taxonomic and Geographic Reference. Johns Hopkins University Press, Baltimore, pp 38–42
Groves CP, Flannery T (1990) Revision of the families and genera of bandicoots. In: Seebeck JH, Brown PR, Wallis RL, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty & Sons, Baulkham Hills, New South Wales, pp 1–11
Hershkovitz, P (1995) The staggered marsupial third lower incisor: hallmark of cohort Didelphimorphia, and description of a new genus and species with staggered i3 from the Albian (Lower Cretaceous) of Texas. Bonn Zool Beitr 45:153–169
Horovitz I, Sánchez-Villagra MR (2003) A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics 19:181–212. doi:10.1111/j.1096-0031.2003.tb00363.x
Hughes RL, Hall LS, Archer M, Aplin K (1990) Observations on placentation and development in Echymipera kalubu. In: Seebeck JH, Brown PR, Wallis RL, Kemper CM (eds) Bandicoots and Bilbies. Surrey Beatty & Sons, Baulkham Hills, New South Wales, pp 259–270
Jolicoeur P (1963) The multivariate generalization of the allometry equation. Biometrics 19:497–499. doi:10.2307/2527939
Kingsmill E (1962) An investigation of criteria for estimating age in the marsupials Trichosurus vulpecula Kerr and Perameles nasuta Geoffroy. Austral J Zool 10:597–616. doi:10.1071/ZO9620597
Kirsch JAW, Waller PF (1979) Notes on the trapping and behavior of the Caenolestidae (Marsupialia). J Mammal 60:390–395. doi:10.2307/1379811
Kirsch JAW, Reig OA, Springer MS (1991) DNA hybridization evidence for the Australian affinity of the American marsupial Dromiciops australis. PNAS 88:10465–10469.
Koyabu DD, Werneburg I, Morimoto N, Zollikofer CPE, Forasiepi AM, Endo H, Kimura J, Ohdachi SD, Son NT, Sánchez-Villagra MR (2014) Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat Comm 5:3625. doi:10.1038/ncomms4625
Krajewski C, Buckley L, Westerman M (1997) DNA phylogeny of the marsupial wolf resolved. Proc Roy Soc Lond 264:911–917. doi:10.1098/rspb.1997.0126
Laurin M (2004) The evolution of body size, Cope’s rule and the origin of amniotes. Syst Biol 53:594–622. doi:10.1080/10635150490445706
Lavedèze S, Muizon C de (2010) Evidence of early evolution of Australidelphia (Metatheria, Mammalia) in South America: phylogenetic relationships of the metatherians from the late Palaeocene of Itaboraí (Brazil) based on teeth and petrosal bones. Zool J Linn Soc 159:746–784. doi:10.1111/j.1096-3642.2009.00577.x
Luckett PW, Hong N (2000) Ontogenetic evidence for dental homologies and premolar replacement in fossil and extant caenolestids (Marsupialia). J Mammal Evol 7:109–127. doi:10.1023/A:1009406632509
Luteyn JL (1999). Páramos: A Checklist of Plant Diversity, Geographical Distribution, and Botanical Literature. New York Botanical Garden Press, New York
Lyne AG (1964) Observations on the breeding and growth of the marsupial Perameles nasuta Geoffroy, with notes on other bandicoots. Austral J Zool 12:322–339. doi:10.1071/ZO9640322#sthash.oG7tuTG7.dpuf
Lyne AG, Mort PA (1981) A comparison of skull morphology in the marsupial bandicoot genus Isoodon: its taxonomic implication and notes on a new species, Isoodon arnhemensis. Austral Mammal 4:107–133
Manly BFJ (1997) Randomization, Bootstrap, and Monte Carlo Methods in Biology, 2nd ed. Chapman & Hall, London
Marshall LG (1980) Systematics of the South American marsupial family Caenolestidae. Fieldiana Geol (n ser) 5:1–145. doi:10.5962/bhl.title.3314
Martin GM (2007) Dental anomalies in Dromiciops gliroides (Microbiotheria, Microbiotheriidae), Caenolestes fuliginosus and Rhyncholestes raphanurus (Paucituberculata, Caenolestidae). Rev Chil Hist Nat 80:393–406. doi:10.4067/S0716-078X2007000400001
Martin GM (2011) Geographic distribution of Rhyncholestes raphanurus Osgood, 1924 (Paucituberculata: Caenolestidae), an endemic marsupial of the Valdivian temperate rainforest. Austral J Zool 59:118–126. doi:10.1071/ZO11038
Martin GM (2013) Intraspecific variability in Lestoros inca (Paucituberculata, Caenolestidae), with reports on dental anomalies and eruption pattern. J Mammal 94:601–617. doi:10.1644/12-MAMM-A-180.1
Maunz M, German Z (1996) Craniofacial heterochrony and sexual dimorphism in the short-tailed opossum (Monodelphis domestica). J Mammal 77:992–1005. doi:10.2307/1382780
Medina CE, Zeballos H, López E (2012) Diversidad de mamíferos en los bosques montanos del valle de Kcosnipata, Cusco, Perú. Mastozool Neotrop 19:345–351. http://www.sarem.org.ar/wp-content/uploads/2012/11/SAREM_MastNeotrop_19-1_08_Medina.pdf
Meredith RW, Westerman M, Springer MS (2008) A timescale and phylogeny for “bandicoots” (Peramelemorphia: Marsupialia) based on sequences for five nuclear genes. Mol Phylogen Evol 47:1–20. doi:10.1016/j.ympev.2008.01.002
Munemasa M, Nikaido M, Donnellan S, Austin CC, Okada N, Hasegawa M (2006) Phylogenetic analysis of diprotodontian marsupials based on complete mitochondrial genomes. Genes Genet Syst 81:181–191. doi:10.1266/ggs.81.181
Myers P, Patton J (2008) Genus Lestoros Oehser, 1934. In: Gardner A (ed) The Mammals of South America, Vol. 1. University of Chicago Press, Chicago, pp 124–126
Nilsson MA, Arnason U, Spencer PBS, Janke A (2004) Marsupial relationships and a timeline for marsupial radiation in south Gondwana. Gene 340:189–196. doi:10.1016/j.gene.2004.07.040
Ojala-Barbour RC, Pinto M, Brito J, Albuja L, Lee TE Jr, Patterson BD (2013) A new species of shrew-opossum (Paucituberculata: Caenolestidae) with a phylogeny of extant caenolestids. J Mammal 94:967–982. doi:10.1644/13-MAMM-A-018.1
Osgood WH (1921) A monographic study of the American marsupial, Caenolestes. Field Mus Nat Hist Zool Ser 14:1–156
Osgood WH (1924) Review of living caenolestids with description of a new genus from Chile. Field Mus Nat Hist Zool Ser 14:165–73. doi:10.5962/bhl.title.2955
Osgood WH (1943) The mammals of Chile. Field Mus Nat Hist Zool Ser 30:1–268.
Palma RE (2003) Evolution of American marsupials and their phylogenetic relationships with Australian metatherians. In: Jones M, Dickman C, Archer M (eds) Predators with Pouches: the Biology of Carnivorous Marsupials. CSIRO Publishing, Collingwood, Australia, pp 21–29
Palma RE, Spotorno AE (1999) Molecular systematic of marsupials based on the rRNA 12S mitochondrial gene: the phylogeny of Didelphimorphia and the living fossil microbiotheriid Dromiciops gliroides Thomas. Mol Phylogen Evol 13:525–535. doi:10.1006/mpev.1999.0678
Patterson BD (2008) Order Paucituberculata Ameghino, 1894. In: Gardner AL (ed) Mammals of South America. Vol. 1. Marsupials, Xenarthrans, Shrews, and Bats. University of Chicago Press, Chicago, pp 119–120
Patterson BD, Gallardo MH (1987) Rhyncholestes raphanurus. Mammal Species 286:1–5
Pearson OP (1995) Annotated keys for identifying small mammals living in or near Nahuel Huapi National Park or Lanin National Park, southern Argentina. Mastozool Neotrop 2:99–148. http://www.sarem.org.ar/wp-content/uploads/2014/05/SAREM_MastNeotrop_2-2_02_Pearson.pdf
Porto A, Shirai, LT, de Oliveira FB, Marroig G (2013) Size variation, growth strategies, and the evolution of modularity in the mammalian skull. Evolution 67:3305–3322. doi:10.1111/evo.12177
Prevosti F, Turazzini CF, Chemisquy MA (2010) Morfología craneana en tigres dientes de sable: alometría, función y filogenia. Ameghiniana 47:239–256. http://www.ameghiniana.org.ar/index.php/ameghiniana/article/view/167
Retief JD, Krajewski C, Westerman M, Winkfein RJ, Dixon GH (1995) Molecular phylogeny and evolution of marsupial protamine PI genes. Proc Roy Soc Lond B 259:7–14. doi:10.1098/rspb.1995.0002
Rougier GW, Apesteguía S, Gaetano LC (2011) Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479: 98–102. doi:10.1038/nature10591
Rougier, GW, Wible JR, Beck RMD, Apesteguía S (2012) The Miocene mammal Necrolestes demonstrates the survival of a Mesozoic nontherian lineage into the late Cenozoic of South America. Proc Natl Acad Sci USA 109(49):20053–20058. doi:10.1073/pnas.1212997109
Sánchez-Villagra MR (2013) Why are there fewer marsupials than placentals? On the relevance of geography and physiology to evolutionary patterns of mammalian diversity and disparity. J Mammal Evol 20:279–290. doi:10.1007/s10914-012-9220-3
Segura V (2014) Ontogenia craneana postnatal en cánidos y félidos neotropicales: funcionalidad y patrones evolutivos. Dissertation, Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina.
Segura V, Prevosti F (2012) A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131:79–92. doi:10.1007/s00435-012-0145-4
Segura V, Prevosti F, Cassini G (2013) Cranial ontogeny in the Puma lineage, Puma concolor, Herpailurus yagouaroundi, and Acinonyx jubatus (Carnivora: Felidae): a three dimensional geometric morphometric approach. Zool J Linn Soc 169:235–250. doi:10.1111/zoj.12047
Shirai L, Marroig G (2010) Skull modularity in neotropical marsupials and monkeys: size variation and evolutionary constraint and flexibility. J Exp Zool 314B:663–683. doi:10.1002/jez.b.21367
Smith RJ (1981) On the definition of variables in studies of primate dental allometry. Am J Phys Anthropol 55:323–329. doi:10.1002/ajpa.1330550306
Smith KK (1997) Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution 51:1663–1678
Solari S, Muñoz-Saba Y, Rodríguez-Mahecha JV, Defler TR, Ramírez-Chaves HE, Trujillo F (2013) Riqueza, endemismo y conservación de los mamíferos de Colombia. Mastozool Neotrop 20:301–365. http://www.sarem.org.ar/wp-content/uploads/2013/12/SAREM_MastNeotrop_20-2_08_Solari.pdf
Springer MS, Westerman M, Kavanagh JR, Burk A, Woodburne MO, Kao DJ, Krajewski C (1998) The origin of the Australasian marsupial fauna and the phylogenetic affinities of the enigmatic monito del monte and marsupial mole. Proc R Soc B 265:2381–2386. doi:10.1098/rspb.1998.0587
Strait SG (1993) Molar morphology and food texture among small bodied faunivorous mammals. J Mammal 74:391–402. doi:10.2307/1382395
Szalay FS (1982) A new appraisal of marsupial phylogeny and classification. In: Archer M (ed) Carnivorous Marsupials. Royal Society of New South Wales, Crow Nest, pp 621–640
Szalay FS (1994) Evolutionary History of the Marsupials and an Analysis of Osteological Characters. Cambridge University Press, New York
Szalay FS, Sargis EJ (2001) Model-based analysis of poscranial osteology of marsupials of Palaeocene of Itaboraí (Brazil) and the phylogenetics and biogeography of Metatheria. Geodiversitas 23: 139–302
Tarnawski BA, Cassini GH, Flores DA (2014) Allometry of the postnatal cranial ontogeny and sexual dimorphism in Otaria byronia (Otariidae). Acta Theriol 59:81–97. doi:10.1007/s13364-012-0124-7
Tarnawski BA, Cassini GH, Flores DA (2014) Skull allometry and sexual dimorphism in the ontogeny of the southern elephant seal (Mirounga leonina). Can J Zool 92:19–31. doi10.1139/cjz-2013-0106
Temple-Smith PD (1987) Sperm structure and marsupial phylogeny. In: Archer M (ed) Possums and Opossums: Studies in Evolution. Surrey Beatty & Sons, Sydney, pp 171–194
Timm RM, Patterson BD (2008) Genus Caenolestes O. Thomas 1895. In: Gardner AL (ed), Mammals of South America. Vol. 1. Marsupials, Xenarthrans, Shrews, and Bats. University of Chicago Press, Chicago, pp 120–124
Travouillon KJ, Archer M, Hand SJ, Muirhead J (2014) Sexually dimorphic bandicoots (Marsupialia: Peramelemorphia) from the Oligo-Miocene of Australia, first cranial ontogeny for fossil bandicoots and new species descriptions. J Mammal Evol. doi:10.1007/s10914-014-9271-8
Tyndale-Biscoe CH (2005) Life of Marsupials. CSIRO Publishing, Sydney
Tyndale-Biscoe H, Renfree MB (1987) Reproductive Physiology of Marsupials. Cambridge University Press, New York
Warton DI, Weber NC (2002) Common slope tests for bivariate structural relationships. Biometrics 44:161–174. doi:10.1002/1521-4036(200203)44:2<161::AID-BIMJ161>3.0.CO;2-N
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line fitting methods for allometry. Biol Rev 81:259–291. doi:10.1017/S1464793106007007
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40:243–261
Wilson L (2011) Comparison of prenatal and postnatal ontogeny: cranial allometry in the African striped mouse (Rhabdomys pumilio). J Mammal 92:407–420. doi:10.1644/10-MAMM-A-209.1
Wilson L (2013) Allometric disparity in rodent evolution. Ecol Evol 3:971–984
Wilson L, Sánchez-Villagra MR (2010) Diversity trends and their ontogenetic basis: an exploration of allometric disparity in rodents. Proc Roy Soc B 277:1227–1234
Wroe S, Milne N (2007) Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution 61:1251–1260
Acknowledgments
We thank curators who allowed the examination of the material under their care: Bruce Patterson and Bill Stanley of the Field Museum of Natural History (FMNH, Chicago), Kris Helgen, Darrin Lunde, and Linda Gordon of the Smithsonian Institution (USMNH, Washington, D.C.), Hugo López and Catalina Cárdenas of the Instituto de Ciencias Naturales Universidad Nacional de Colombia (ICN, Bogotá), and Sergio Solari of the Instituto de Biologia, Universidad de Antioquia (CTUA, Medellín). We thank Valentina Segura and Francisco Prevosti for discussion about character optimization on confidence intervals and for providing raw data of L. culpaeus. Guillermo Cassini performed the R script for bivariate comparisons. GMM thanks E. Watkins and M. Simeon for their economic support. This work was supported by an external fellowship of the Consejo Nacional de Investigaciones Cientificas y Técnicas of Argentina (CONICET) to DAF, and the projects PICT2008-1798 to NPG and PICT2012-1583 to DAF, of the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (ANPCyT).
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 28 kb)
Rights and permissions
About this article
Cite this article
Flores, D.A., Abdala, F., Martin, G.M. et al. Post-Weaning Cranial Growth in Shrew Opossums (Caenolestidae): A Comparison with Bandicoots (Peramelidae) and Carnivorous Marsupials. J Mammal Evol 22, 285–303 (2015). https://doi.org/10.1007/s10914-014-9279-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-014-9279-0