Abstract
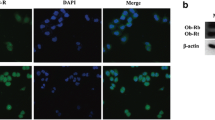
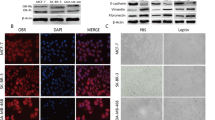
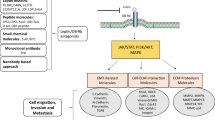
Leptin is a cytokine-like hormone that functions as a link between obesity and breast cancer (BC). Leptin treatment induces Epithelial to Mesenchymal Transition (EMT) in BC cell lines. In non-tumoral breast epithelial MCF10A cells, acute leptin treatment induces partial EMT. However, the effect of chronic leptin treatment on EMT in non-tumorigenic breast cells has not been fully explored. This study aimed to evaluate the effect of chronic leptin treatment on the induction of EMT in MCF10A cells. We found that chronic leptin treatment induces a switch from an epithelial to a mesenchymal morphology, partial loss of E-cadherin and gain of vimentin expression. Immunolocalization experiments showed a partial loss of E-cadherin at cell junctions and increased cytoplasmic localization of vimentin in leptin-treated cells. Moreover, chronic leptin treatment increased collective cell migration and invasion. Furthermore, when cultured in non-adherent conditions leptin treated cells exhibited reduced cell aggregation, increased survival, and decreased apoptosis, which correlates with increased FAK and AKT phosphorylation. Finally, bioinformatic analysis in two publicly available RNAseq datasets from normal breast tissue shows that high levels of leptin mRNA correlate positively with the expression of mesenchymal markers, and negatively with epithelial markers. Thus, our results demonstrate that chronic leptin treatment induces EMT in non-tumorigenic MCF10A cells and suggest that high leptin expression in normal breast tissue may induce EMT and contribute to increased risk of breast cancer.








Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed in this work, and detailed experimental procedures are available upon request.
References
Nattenmüller CJ, Kriegsmann M, Sookthai D, Fortner RT, Steffen A, Walter B, et al. Obesity as risk factor for subtypes of breast cancer: Results from a prospective cohort study. BMC Cancer. 2018;18(1):1–8.
Feigelson HS, Bodelon C, Powers JD, Curtis RE, Buist DSM, Veiga LHS, et al. Body Mass Index and Risk of Second Cancer among Women with Breast Cancer. J Natl Cancer Inst. 2021;113(9):1156–60.
Blair CK, Wiggins CL, Nibbe AM, Storlie CB, Prossnitz ER, Royce M, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5(1):1–7.
Bhardwaj P, Brown KA. Obese Adipose Tissue as a Driver of Breast Cancer Growth and Development: Update and Emerging Evidence. Front Oncol. 2021;11:638918.
Wu Q, Li B, Li Z, Li J, Sun S, Sun S. Cancer-associated adipocytes: Key players in breast cancer progression. J Hematol Oncol. 2019;12(1):1–5.
Andò S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, et al. Obesity, leptin and breast cancer: Epidemiological evidence and proposed mechanisms. Cancers. 2019;11(1):62.
Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and Breast Cancer: Role of Leptin. Front Oncol. 2019;9:596.
Bahathiq S, Omar A. Relationship of leptin hormones with body mass index and waist circumference in Saudi female population of the Makkah Community. Open Obes J. 2010;2(1):95–100.
Harris RBS. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta Mol Basis Dis. 2014;1842(3):414–23.
Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The Association between Leptin Level and Breast Cancer: A Meta-Analysis. PLoS One. 2013;8(6):e67349.
Mohammadzadeh G, Ghaffari MA, Bafandeh A, Hosseini SM. Association of serum soluble leptin receptor and leptin levels with breast cancer. Int J Res Med Sci. 2014;19(5):433.
Gu L, Di WC, Cao C, Cai LR, Li DH, Zheng YZ. Association of serum leptin with breast cancer. A meta-analysis Medicine (Baltimore). 2019;98(5):e14094.
Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: Possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–53.
Karaduman M, Bilici A, Ozet A, Sengul A, Musabak U, Alomeroglu M. Tissue leptin levels in patients with breast cancer. J BUON. 2010;15(2):369–72.
Xu M, le Cao F, Li N, Gao X, Su X, Jiang X. Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol Lett. 2018;16(4):4782–8.
Zhang B, Chen X, Xie C, Chen Z, Liu Y, Ru F, et al. Leptin promotes epithelial-mesenchymal transition in benign prostatic hyperplasia through downregulation of BAMBI. Exp Cell Res. 2020;387(1):111754.
Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via AKT/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287(11):8598–612.
Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, et al. Activation of IL-8 via PI3K/AKT-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16(8):1220–30.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol. 2019;20(2):69–84.
Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol. 2020;21(6):341–52.
Wei L, Li K, Pang X, Guo B, Su M, Huang Y, et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res. 2016;35(1):1–10.
Villanueva-Duque A, Zuniga-Eulogio MD, Dena-Beltran J, Castaneda-Saucedo E, Calixto-Galvez M, Mendoza-Catalán MA, et al. Leptin induces partial epithelial-mesenchymal transition in a FAK-ERK dependent pathway in MCF10A mammary non-tumorigenic cells. Int J Clin Exp Pathol. 2017;10(10):10334–42.
Olea-Flores M, Zuñiga-Eulogio M, Tacuba-Saavedra A, Bueno-Salgado M, Sánchez-Carvajal A, Vargas-Santiago Y, et al. Leptin Promotes Expression of EMT-Related Transcription Factors and Invasion in a SRC and FAK-Dependent Pathway in MCF10A Mammary Epithelial Cells. Cells. 2019;8(10):1133.
Hosney M, Sabet S, El-Shinawi M, Gaafar KM, Mohamed MM. Leptin is overexpressed in the tumor microenvironment of obese patients with estrogen receptor positive breast cancer. Exp Ther Med. 2017;13(5):2235–46.
Mishra AK, Parish CR, Wong M-L, Licinio J, Blackburn AC. Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS One. 2017;12(5):e0178454.
Bowers LW, Rossi EL, McDonell SB, Doerstling SS, Khatib SA, Lineberger CG, et al. Leptin signaling mediates obesity-associated CSC enrichment and EMT in preclinical TNBC models. Mol Cancer Res. 2018;16(5):869–79.
Kang T, Yau C, Wong CK, Sanborn JZ, Newton Y, Vaske C, et al. A risk-associated Active transcriptome phenotype expressed by histologically normal human breast tissue and linked to a pro-tumorigenic adipocyte population. Breast Cancer Res. 2020;22(1):1–15.
Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8. https://doi.org/10.1038/s41587-020-0546-8.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645–54.
Moreno-Bueno G, Peinado H, Molina P, Olmeda D, Cubillo E, Santos V, et al. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nat Protoc. 2009;4(11):1591–613.
Peixoto P, Etcheverry A, Aubry M, Missey A, Lachat C, Perrard J, et al. EMT is associated with an epigenetic signature of ECM remodeling genes. Cell Death Dis. 2019;10(3):1–17.
Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24(6):1838–51.
Liu CY, Lin HH, Tang MJ, Wang YK. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6(18):15966–83.
Leggett SE, Hruska AM, Guo M, Wong IY. The epithelial-mesenchymal transition and the cytoskeleton in bioengineered systems. Cell Commun Signal. 2021;19(1):1–24.
Pastushenko I, Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019;29(3):212–26.
Wong IY, Javaid S, Wong EA, Perk S, Haber DA, Toner M, et al. Collective and individual migration following the epithelial-mesenchymal transition. Nat Mater. 2014;13(11):1063–71.
Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev Cell. 2018;45(6):681-95.e4.
Røsland GV, Dyrstad SE, Tusubira D, Helwa R, Tan TZ, Lotsberg ML, et al. Epithelial to mesenchymal transition (EMT) is associated with attenuation of succinate dehydrogenase (SDH) in breast cancer through reduced expression of SDHC. Cancer Metab. 2019;7(1):1–18.
Lourenço AR, Roukens MG, Seinstra D, Frederiks CL, Pals CE, Vervoort SJ, et al. C/EBPɑ is crucial determinant of epithelial maintenance by preventing epithelial-to-mesenchymal transition Nat. Commun. 2020;11(1):1–18.
Huang RYJ, Wong MK, Tan TZ, Kuay KT, Ng AH, Chung VY, et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis. 2013;4(11):e915.
Wang D, Zhang L, Hu A, Wang Y, Liu Y, Yang J, et al. Loss of 4.1N in epithelial ovarian cancer results in EMT and matrix-detached cell death resistance. Protein Cell. 2021;12(2):107–27.
Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, et al. Fak/SRC signaling in human intestinal epithelial cell survival and anoikis: Differentiation state-specific uncoupling with the PI3-K/AKT-1 and MEK/Erk pathways. J Cell Physiol. 2007;212(3):717–28.
Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta Mol Cell Res. 2013;1833(12):3481–98.
Yang J, Hou Y, Zhou M, Wen S, Zhou J, Xu L, et al. Twist induces epithelial-mesenchymal transition and cell motility in breast cancer via ITGB1-FAK/ILK signaling axis and its associated downstream network. Int J Biochem Cell Biol. 2016;71:62–71.
Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68(4):989–97.
Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan BY, et al. Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells. PLoS One. 2015;10(7):e0131285.
Gould R, Bassen DM, Chakrabarti A, Varner JD, Butcher J. Population Heterogeneity in the Epithelial to Mesenchymal Transition Is Controlled by NFAT and Phosphorylated Sp1. PLoS Comput Biol. 2016;12(12):e1005251.
Acheva A, Kärki T, Schaible N, Krishnan R, Tojkander S. Adipokine Leptin Co-operates With Mechanosensitive Ca2 +-Channels and Triggers Actomyosin-Mediated Motility of Breast Epithelial Cells. Front Cell Dev Biol. 2021;8:607038.
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, et al. G9a interacts with Snail and is critical for Snail-mediated E-cadherin repression in human breast cancer. J Clin Investig. 2012;122(4):1469–86.
Meyer-Schaller N, Cardner M, Diepenbruck M, Saxena M, Tiede S, Lüönd F, et al. A Hierarchical Regulatory Landscape during the Multiple Stages of EMT. Dev Cell. 2019;48(4):539-53.e6.
Chandrasekaran B, Dahiya NR, Tyagi A, Kolluru V, Saran U, Baby BV, et al. Chronic exposure to cadmium induces a malignant transformation of benign prostate epithelial cells. Oncogenesis. 2020;9(2):1–10.
Hartsock A, Nelson WJ. Competitive regulation of E-cadherin juxtamembrane domain degradation by p120-catenin binding and Hakai-mediated ubiquitination. PLoS One. 2012;7(5):e37476.
Fan X, Jin S, Li Y, Khadaroo PA, Dai Y, He L, et al. Genetic and epigenetic regulation of e-cadherin signaling in human hepatocellular carcinoma. Cancer Manag Res. 2019;11:8947–63.
Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–34.
Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–57.
Chen A, Beetham H, Black MA, Priya R, Telford BJ, Guest J, et al. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer. 2014;14(1):1–14.
Juárez-Cruz JC, Zuñiga-Eulogio MD, Olea-Flores M, Castañeda-Saucedo E, Mendoza-Catalán MÁ, Ortuño-Pineda C, et al. Leptin induces cell migration and invasion in a FAK-SRC-dependent manner in breast cancer cells. Endocr Connect. 2019;8(11):1539–52.
Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGFβ signaling and metastasis. Breast Cancer Res. 2009;11(5):1–16.
Bae GY, Hong SK, Park JR, Kwon OS, Kim KT, Koo JH, et al. Chronic TGFβ stimulation promotes the metastatic potential of lung cancer cells by Snail protein stabilization through integrin β3-AKT-GSK3β signaling. Oncotarget. 2016;7(18):25366–76.
Katsuno Y, Meyer DS, Zhang Z, Shokat KM, Akhurst RJ, Miyazono K, et al. Chronic TGFb exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal. 2019;12(570):eaau8544.
Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5(8):733–40.
Collins NL, Reginato MJ, Paulus JK, Sgroi DC, LaBaer J, Brugge JS. G 1 /S Cell Cycle Arrest Provides Anoikis Resistance through Erk-Mediated Bim Suppression. Cell Mol Biol. 2005;25(12):5282–91.
Walker S, Foster F, Wood A, Owens T, Brennan K, Streuli CH, et al. Oncogenic activation of FAK drives apoptosis suppression in a 3D-culture model of breast cancer initiation. Oncotarget. 2016;7(43):70336–52.
Beauséjour M, Noël D, Thibodeau S, Bouchard V, Harnois C, Beaulieu JF, et al. Integrin/Fak/SRC-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: Selective engagement and roles of PI3-K isoform complexes. Apoptosis. 2012;17(6):566–78.
Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, et al. Combinatorial activation of FAK and AKT by transforming growth factor-β1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19(4):761–71.
Román-Pérez E, Casbas-Hernández P, Pirone JR, Rein J, Carey LA, Lubet RA, et al. Gene expression in extratumoral microenvironment predicts clinical outcome in breast cancer patients. Breast Cancer Res. 2012;14(2):1–12.
Troester MA, Lee MH, Carter M, Fan C, Cowan DW, Perez ER, et al. Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res. 2009;15(22):7020–8.
Delort L, Cholet J, Decombat C, Vermerie M, Dumontet C, Castelli FA, et al. The Adipose Microenvironment Dysregulates the Mammary Myoepithelial Cells and CouldParticipate to the Progression of Breast Cancer. Front Cell Dev Biol. 2020;8:571948.
Tenvooren I, Jenks MZ, Rashid H, Cook KL, Muhlemann JK, Sistrunk C, et al. Elevated leptin disrupts epithelial polarity and promotes premalignant alterations in the mammary gland. Oncogene. 2019;38(20):3855–70.
Giordano C, Chemi F, Panza S, Barone I, Bonofiglio D, Lanzino M, et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget. 2016;7(2):1262–75.
Esper RM, Dame M, Mcclintock S, Holt PR, Dannenberg AJ, Swicha M, et al. Leptin and adiponectin modulate the self-renewal of normal human breast epithelial stem cells. Cancer Prev Res (Phila). 2015;8(12):1174–83.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell. 2008;133(4):704–15.
Funding
The project was founded by the “Programa de Fortalecimiento de la Calidad Educativa (PFCE)” awarded to Napoleón Navarro-Tito and Eduardo Castañeda-Saucedo (2018–2020). Juan Carlos Juárez-Cruz was awarded a PhD fellowship from the “Consejo Nacional de Ciencia y Tecnología (CONACYT)”, from 2018–2021.
Author information
Authors and Affiliations
Contributions
JCJC, NNT and ECS contributed to the study conception and design. Material preparation, experimental procedures, and data collection were performed by JCJC. All authors contributed to data analysis and interpretation. JCJC, ECS contributed to RNAseq dataset selection and design of bioinformatic analyses, and MO performed RNAseq bioinformatic analysis and contributed to data interpretation. MR, COP contributed to data analysis and interpretation, and figure preparation. The first draft of the manuscript was written by JCJC and ECS, and all authors contributed to improving the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participation
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juárez-Cruz, J.C., Okoniewski, M., Ramírez, M. et al. Chronic Leptin Treatment Induces Epithelial-Mesenchymal Transition in MCF10A Mammary Epithelial Cells. J Mammary Gland Biol Neoplasia 27, 19–36 (2022). https://doi.org/10.1007/s10911-022-09515-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-022-09515-9