Abstract
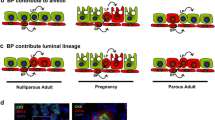
The hormone-sensing mammary epithelial cell (HS-MEC—expressing oestrogen receptor-alpha (ERα) and progesterone receptor (PGR)) is often represented as being terminally differentiated and lacking significant progenitor activity after puberty. Therefore while able to profoundly influence the proliferation and function of other MEC populations, HS-MECs are purported not to respond to sex hormone signals by engaging in significant cell proliferation during adulthood. This is a convenient and practical simplification that overshadows the sublime, and potentially critical, phenotypic plasticity found within the adult HS-MEC population. This concept is exemplified by the large proportion (~80 %) of human breast cancers expressing PGR and/or ERα, demonstrating that HS-MECs clearly proliferate in the context of breast cancer. Understanding how HS-MEC proliferation and differentiation is driven could be key to unraveling the mechanisms behind uncontrolled HS-MEC proliferation associated with ERα- and/or PGR-positive breast cancers. Herein we review evidence for the existence of a HS-MEC progenitor and the emerging plasticity of the HS-MEC population in general. This is followed by an analysis of hormones other than oestrogen and progesterone that are able to influence HS-MEC proliferation and differentiation: androgens, prolactin and transforming growth factor-beta1.



Similar content being viewed by others
Abbreviations
- ALDH:
-
Alcohol Dehydrogenase
- AREG:
-
Aregulin
- AR:
-
Androgen Receptor
- BER-EP4:
-
Epithelial-Specific Antigen (also known as EPCAM)
- CALLA:
-
Common Acute Lymphoblastic Leukaemia antigen (also known as CD10)
- C/EBPβ:
-
CAAT/Enhancer Binding Protein
- CD49b:
-
Cluster of Differentiation 49b (also known as alpha-2 Integrin)
- CD49f:
-
Cluster of Differentiation 49f (also known as alpha-6 Integrin)
- ELF5:
-
E74-like Protein 5
- EGF:
-
Epidermal Growth Factor
- EMT:
-
Epithelial to Mesenchymal Transition
- FACS:
-
Fluorescence-Activated Cell Sorting
- FOXA1:
-
Forkhead box A1
- GATA3:
-
GATA-binding protein 3
- GREB1:
-
Growth Regulated by Estrogen in the Breast 1
- GH:
-
Growth Hormone
- HS-MECs:
-
Hormone-Sensing Mammary Epithelial Cells
- IGF2:
-
Insulin-like Growth Factor 2
- JAK2:
-
Janus Kinase 2
- LRH1:
-
Liver Receptor Homolog 1
- LA:
-
Lobuloalveolar Unit
- MMPs:
-
Matrix Metalloproteinases
- MMTV:
-
Mouse Mammary Tumour Virus
- MUC1:
-
Mucin 1
- ERα:
-
Oestrogen Receptor-alpha
- PELP1:
-
Proline, Glutamate and Leucine Rich Protein 1
- PGR:
-
Progesterone Receptor
- PRLR:
-
Prolactin Receptor
- RANKL:
-
Receptor Activator of Nuclear factor Kappa-B Ligand
- RUNX:
-
RUNT-related Transcription Factor
- SCA1:
-
Stem Cells Antigen 1 (also known as LY6A/E)
- SMAD:
-
Mothers Against Decapentaplegic
- SP:
-
Side Population
- STAT5:
-
Signal Transducer and Activator of Transcription 5
- TBX3:
-
T-box Transcription Factor 3
- TDLU:
-
Terminal Ductal Lobule Unit
- TOX3:
-
TOX High Mobility Group Box Family Member 3
- TGFβ:
-
Transforming Growth Factor-beta
- WAP:
-
Whey-Acidic Protein
- WNT:
-
Wingless-type MMTV Integration Site Family Member
References
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–4.
Aupperlee MD, Leipprandt JR, Bennett JM, Schwartz RC, Haslam SZ. Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res. 2013;15:R44.
Mukherjee A, Soyal SM, Li J, Ying Y, He B, DeMayo FJ, et al. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J. 2010;24:4408–19.
Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat M-L, et al. Generation of a functional mammary gland from a single stem cell. Nat Cell Biol. 2006;439:84–8.
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–7.
Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26.
Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–7.
Shiah Y-J, Tharmapalan P, Casey AE, Joshi PA, McKee TD, Jackson HW, et al. A progesterone-CXCR4 axis controls mammary progenitor cell fate in the adult gland. Stem Cell Rep. 2015;4:313–22.
Mastroianni M, Kim S, Kim YC, Esch A, Wagner C, Alexander CM. Wnt signaling can substitute for estrogen to induce division of ERα-positive cells in a mouse mammary tumor model. Cancer Lett. 2010;289:23–31.
Cardiff RD. Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia. 1998;3:3–5.
Lain AR, Creighton CJ, Conneely OM. Research resource: progesterone receptor targetome underlying mammary gland branching morphogenesis. Mol Endocrinol. 2013;27:1743–61.
Fata JE, Chaudhary V, Khokha R. Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod. 2001;65:680–8.
Robinson GW, McKnight RA, Smith GH, Hennighausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–90.
Going JJ, Anderson TJ, Battersby S, MacIntyre CC. Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol. 1988;130:193–204.
Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58:163–70.
Anderson E, Clarke RB, Howell A. Estrogen responsiveness and control of normal human breast proliferation. J Mammary Gland Biol Neoplasia. 1998;3:23–35.
Hu H, Wang J, Gupta A, Shidfar A, Branstetter D, Lee O, et al. RANKL expression in normal and malignant breast tissue responds to progesterone and is up-regulated during the luteal phase. Breast Cancer Res Treat. 2014;146:515–23.
Cardiff R, Anver M, Boivin G, Bosenberg M, Maronpot R, Molinolo A, et al. Precancer in mice: animal models used to understand, prevent, and treat human precancers. Toxicol Pathol. 2006;34:699–707.
Calaf G, Alvarado M, Bonney G, Amfoh K, Russo J. Influence of lobular development on breast epithelial-cell proliferation and steroid-hormone receptor content. Int J Oncol. 1995;7:1285–8.
Russo J, Russo IH. Development of the human breast. Maturitas. 2004;49:2–15.
Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3:49–61.
Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–27.
Fendrick JL, Raafat AM, Haslam SZ. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia. 1998;3:7–22.
Li S, Han B, Liu G, Li S, Ouellet J, Labrie F, et al. Immunocytochemical localization of sex steroid hormone receptors in normal human mammary gland. J Histochem Cytochem. 2010;58:509–15.
Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–56.
Booth BW, Boulanger CA, Smith GH. Selective segregation of DNA strands persists in long label retaining mammary cells during pregnancy. Breast Cancer Res. 2008;10:R90.
Booth BW, Smith GH. Breast Cancer Res. 2006;8:R49.
Santagata S, Thakkar A, Ergonul A, Wang B, Woo T, Hu R, et al. Taxonomy of breast cancer based on normal cell phenotype predicts outcome. J Clin Invest. 2014;124:859–70.
Shoker BS, Jarvis C, Sibson DR, Walker C, Sloane JP. Oestrogen receptor expression in the normal and pre-cancerous breast. J Pathol. 1999;188:237–44.
Ewan KBR, Oketch-Rabah HA, Ravani SA, Shyamala G, Moses HL, Barcellos-Hoff MH. Proliferation of estrogen receptor-alpha-positive mammary epithelial cells is restrained by transforming growth factor-beta1 in adult mice. Am J Pathol. 2005;167:409–17.
Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107:2989–94.
De Silva D, Kunasegaran K, Ghosh S, Pietersen AM. Transcriptome analysis of the hormone-sensing cells in mammary epithelial reveals dynamic changes in early pregnancy. BMC Dev Biol. 2015;15:a003178.
Tornillo G, Smalley MJ. ERrrr…Where are the progenitors? Hormone receptors and mammary cell heterogeneity. J Mammary Gland Biol Neoplasia. 2015. doi:10.1007/s10911-015-9336-1
Li W, Ferguson BJ, Khaled WT, Tevendale M, Stingl J, Poli V, et al. PML depletion disrupts normal mammary gland development and skews the composition of the mammary luminal cell progenitor pool. Proc Natl Acad Sci. 2009;106:4725–30.
Kunasegaran K, Ho V, Chang TH-T, De Silva D, Bakker ML, Christoffels VM, et al. Transcriptional repressor Tbx3 is required for the hormone-sensing cell lineage in mammary epithelium. PLoS ONE. 2014;9:e110191.
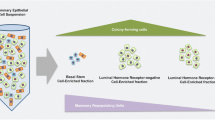
Shehata M, Teschendorff A, Sharp G, Novcic N, Russell IA, Avril S, et al. Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res. 2012;14:R134.
Joshi PA, Waterhouse PD, Kannan N, Narala S, Fang H, Di Grappa MA, et al. RANK signaling amplifies WNT-responsive mammary progenitors through R-SPONDIN1. Stem Cell Rep. 2015;5:31–44.
Choudhury S, Almendro V, Merino VF, Wu Z, Maruyama R, Su Y, et al. Molecular profiling of human mammary gland links breast cancer risk to a p27+ cell population with progenitor characteristics. Cell Stem Cell. 2013;13:117–30.
Honeth G, Lombardi S, Ginestier C, Hur M, Marlow R, Buchupalli B, et al. Aldehyde dehydrogenase and estrogen receptor define a hierarchy of cellular differentiation in the normal human mammary epithelium. Breast Cancer Res. 2014;16:1–14.
Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1pos cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56.
Clayton H, Titley I, Vivanco MD. Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp Cell Res. 2004;297:444–60.
Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco MDM, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–8.
Pellegrini P, Cordero A, Gallego MI, Dougall WC, Purificación M, Pujana MA, et al. Constitutive activation of RANK disrupts mammary cell fate leading to tumorigenesis. Stem Cells. 2013;31:1954–65.
Tarulli GA, De Silva D, Ho V, Kunasegaran K, Ghosh K, Tan BC, et al. Hormone-sensing cells require Wip1 for paracrine stimulation in normal and premalignant mammary epithelium. Breast Cancer Res. 2013;15:R10.
Regan JL, Kendrick H, Magnay F-A, Vafaizadeh V, Groner B, Smalley MJ. c-Kit is required for growth and survival of the cells of origin of Brca1-mutation-associated breast cancer. Oncogene. 2012;31:869–83.
Smith GH, Vonderhaar BK, Graham DE, Medina D. Expression of pregnancy-specific genes in preneoplastic mouse mammary tissues from virgin mice. Cancer Res. 1984;44:3426–37.
Ferguson DJ. Ultrastructural characterisation of the proliferative (stem?) cells within the parenchyma of the normal “resting” breast. Virchows Arch A Pathol Anat Histopathol. 1985;407:379–85.
Chepko G, Smith GH. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239–53.
Smith GH, Chepko G. Mammary epithelial stem cells. Microsc Res Tech. 2001;52:190–203.
Hilton HN, Graham JD, Kantimm S, Santucci N, Cloosterman D, Huschtscha LI, et al. Progesterone and estrogen receptors segregate into different cell subpopulations in the normal human breast. Mol Cell Endocrinol. 2012;361:191–201.
Hilton HN, Doan TB, Graham JD, et al. Acquired convergence of hormone signaling in breast cancer: ER and PR transition from functionally distinct in normal breast to predictors of metastatic disease. Oncotarget. 2014;5(18):8651–64.
Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80:137–48.
Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, et al. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst. 2006;98:1011–4.
Prater MD, Petit V, Alasdair Russell I, Giraddi RR, Shehata M, Menon S, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16:942–50.
Brisken C, Ataca D. Endocrine hormones and local signals during the development of the mouse mammary gland. Wiley Interdiscip Rev Dev Biol. 2015;4(3):181–95.
Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, et al. Progesterone receptor modulates ERα action in breast cancer. Nature. 2015;523:313–7.
Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13:385–96.
Tanos T, Rojo L, Echeverria P, Brisken C. ER and PR signaling nodes during mammary gland development. Breast Cancer Res. 2012;14:210.
Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, et al. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210:96–106.
Miyoshi K. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–42.
Santos SJ, Haslam SZ, Conrad SE. Signal transducer and activator of transcription 5a mediates mammary ductal branching and proliferation in the nulliparous mouse. Endocrinology. 2010;151:2876–85.
Santos SJ, Haslam SZ, Conrad SE. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology. 2008;149:329–38.
Wood CE, Branstetter D, Jacob AP, Cline JM, Register TC, Rohrbach K, et al. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013;15:R62.
Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, et al. Receptor activator of NF- B ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem. 2003;278:46171–8.
Obr AE, Grimm SL, Bishop KA, Pike JW, Lydon JP, Edwards DP. Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol Endocrinol. 2013;27:1808–24.
Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, et al. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–87.
Hovey RC. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol. 2002;17:460–71.
Nevalainen MT, Xie J, Bubendorf L, Wagner K-U, Rui H. Basal activation of transcription factor signal transducer and activator of transcription (Stat5) in nonpregnant mouse and human breast epithelium. Mol Endocrinol (Baltimore, Md). 2002;16:1108–24.
Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, et al. Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol Endocrinol. 2007;21:106–25.
Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, et al. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163–75.
O’Leary KA, Jallow F, Rugowski DE, Sullivan R, Sinkevicius KW, Greene GL, et al. Prolactin activates ER in the absence of ligand in female mammary development and carcinogenesis in vivo. Endocrinology. 2013;154:4483–92.
Dong J, Tong T, Reynado AM, Rosen JM, Huang S, Li Y. Dev Biol. 2010;346:196–203.
Forsbach G, Güitrón-Cantú A, Vázquez-Lara J, Mota-Morales M, Díaz-Mendoza ML. Virilizing adrenal adenoma and primary amenorrhea in a girl with adrenal hyperplasia. Arch Gynecol Obstet. 2000;263:134–6.
Hickey TE, Robinson JLL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol (Baltimore, Md). 2012;26:1252–67.
Peters AA, Ingman WV, Tilley WD, Butler LM. Differential effects of exogenous androgen and an androgen receptor antagonist in the peri- and postpubertal murine mammary gland. Endocrinology. 2011;152:3728–37.
Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J Off Publ Fed Am Soc Exp Biol. 2000;14:1725–30.
Gao YRE, Walters KA, Desai R, Zhou H, Handelsman DJ, Simanainen U. Androgen receptor inactivation resulted in acceleration in pubertal mammary gland growth, upregulation of ERα expression, and Wnt/β-catenin signaling in female mice. Endocrinology. 2014;155:4951–63.
Yeh S. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med. 2003;198:1899–908.
Eigeliene N, Elo T, Linhala M, Hurme S, Erkkola R, Harkonen P. Androgens inhibit the stimulatory action of 17-estradiol on normal human breast tissue in explant cultures. J Clin Endocrinol Metab. 2012;97:E1116–27.
Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G. Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor alpha in luminal breast cancer cells. Mol Endocrinol. 2012;26:1941–52.
Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–40.
Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: a novel therapeutic target for hormonal cancers. IUBMB Life. 2010;62:162–9.
Girard BJ, Daniel AR, Lange CA, Ostrander JH. PELP1: a review of PELP1 interactions, signaling, and biology. Mol Cell Endocrinol. 2014;382:642–51.
Lanzino M, Maris P, Sirianni R, Barone I, Casaburi I, Chimento A, et al. DAX-1, as an androgen-target gene, inhibits aromatase expression: a novel mechanism blocking estrogen- dependent breast cancer cell proliferation. Cell Death Dis. 2013;4:e724.
Wong MM, Guo C, Zhang J. Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. Am J Clin Exp Urol. 2014;2:169–87.
Wang X, Yarid N, McMahon L, Yang Q, Hicks DG. Expression of androgen receptor and its association with estrogen receptor and androgen receptor downstream proteins in normal/benign breast luminal epithelium. Appl Immunohistochem Mol Morphol. 2014;22:498–504.
Tarulli GA, Butler LM, Tilley WD, Hickey TE. Bringing androgens up a NOTCH in breast cancer. Endocr Relat Cancer. 2014;21:T183–202.
Ramakrishnan R, Khan SA, Badve S. Morphological changes in breast tissue with menstrual cycle. Mod Pathol. 2002;15:1348–56.
Ferguson DJ, Anderson TJ. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981;44:177–81.
Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104:23–34.
Thomson AA, Marker PC. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation. 2006;74:382–92.
Crane JL, Cao X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J Clin Invest. 2014;124:466–72.
Hameedaldeen A, Liu J, Batres A, Graves G, Graves D. FOXO1, TGF-β regulation and wound healing. IJMS. 2014;15:16257–69.
Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76.
Jenkins G. The role of proteases in transforming growth factor-β activation. Int J Biochem Cell Biol. 2008;40:1068–78.
Wipff P, Hinz B. Integrins and the activation of latent transforming growth factor β1—an intimate relationship. Eur J Cell Biol. 2008;87:601–15.
Moses H, Barcellos-Hoff MH. TGF-biology in mammary development and breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003277.
Barcellos-Hoff M, Akhurst RJ. Transforming growth factor-β in breast cancer: too much, too late. Breast Cancer Res. 2009;11:202.
Wrana JL. Signaling by the TGF-beta superfamily. Cold Spring Harb Perspect Biol. 2013;5:a011197.
Silberstein GB, Daniel CW. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science. 1987;237:291–3.
Zugmaier G, Lippman ME. Effects of TGF beta on normal and malignant mammary epithelium. Ann N Y Acad Sci. 1990;593:272–5.
Pierce DF, Johnson MD, Matsui Y, Robinson SD, Gold LI, Purchio AF, et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 1993;7:2308–17.
Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-β or Wnt5a results in an increase in Wnt/β-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009;11:R19.
Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-mediated inhibition of ductal growth. Development. 2007;134:3929–39.
Lazarus KA, Brown KA, Young MJ, Zhao Z, Coulson RS, Chand AL, et al. Conditional overexpression of liver receptor homolog-1 in female mouse mammary epithelium results in altered mammary morphogenesis via the induction of TGF-β. Endocrinology. 2014;155:1606–17.
Chand AL, Wijayakumara DD, Knower KC, Herridge KA, Howard TL, Lazarus KA, et al. The orphan nuclear receptor LRH-1 and ERα activate GREB1 expression to induce breast cancer cell proliferation. PLoS ONE. 2012;7:e31593.
Mohammed H, D’Santos C, Serandour AA, Ali HR, Brown GD, Atkins A, et al. Endogenous purification reveals GREB1as a key estrogen receptor regulatory factor. Cell Rep. 2013;3:342–9.
Itman C, Wong C, Hunyadi B, Ernst M, Jans DA, Loveland KL. Smad3 dosage determines androgen responsiveness and sets the pace of postnatal testis development. Endocrinology. 2011;152:2076–89.
Justulin Jr LA, Della-Coleta HHM, Taboga SR, Felisbino SL. Matrix metalloproteinase (MMP)-2 and MMP-9 activity and localization during ventral prostate atrophy and regrowth. Int J Androl. 2010;33:696–708.
Cocolakis E, Dai M, Drevet L, Ho J, Haines E, Ali S, et al. Smad signaling antagonizes STAT5-mediated gene transcription and mammary epithelial cell differentiation. J Biol Chem. 2007;283:1293–307.
Chuang LSH, Ito K, Ito Y. RUNX family: regulation and diversification of roles through interacting proteins. Int J Cancer. 2012;132:1260–71.
Chang TH-T, Kunasegaran K, Tarulli GA, De Silva D, Voorhoeve PM, Pietersen AM. New insights into lineage restriction of mammary gland epithelium using parity-identified mammary epithelial cells. Breast Cancer Res. 2014;16:R1.
Jhappan C, Geiser AG, Kordon EC, Bagheri D, Hennighausen L, Roberts AB, et al. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993;12:1835–45.
Kordon EC, McKnight RA, Jhappan C, Hennighausen L, Merlino G, Smith GH. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995;168:47–61.
Boulanger CA, Wagner K-U, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-β1 expression. Oncogene. 2004;24:552–60.
Booth BW, Jhappan C, Merlino G, Smith GH. TGFβ1 and TGFα contrarily affect alveolar survival and tumorigenesis in mouse mammary epithelium. Int J Cancer. 2006;120:493–9.
Muraoka-Cook RS, Kurokawa H, Koh Y, Forbes JT, Roebuck LR, Barcellos-Hoff MH, et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–11.
Muraoka RS, Koh Y, Roebuck LR, Sanders ME, Brantley-Sieders D, Gorska AE, et al. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor 1. Mol Cell Biol. 2003;23:8691–703.
Asselin-Labat M-L, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2006;9:201–9.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–55.
Kouros-Mehr H, Kim J-W, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20:164–70.
Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. Hurtado 2011-NatGen. Nat Genet. 2010;43:27–33.
Theodorou V, Stark R, Menon S, Carroll JS. GATA3 acts upstream of FOXA1 in mediating ESR1 binding by shaping enhancer accessibility. Genome Res. 2013;23:12–22.
Bernardo GM, Keri RA. FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep. 2011;32:113–30.
Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O’Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97:6379–84.
Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, et al. FOXA1 is an essential determinant of ER-alpha expression and mammary ductal morphogenesis. Development. 2010;137:2045–54.
Robinson JLL, Carroll JS. FoxA1 is a key mediator of hormonal response in breast and prostate cancer. Front Endocrinol (Lausanne). 2012;3:68.
Andres SA, Wittliff JL. Co-expression of genes with estrogen receptor-α and progesterone receptor in human breast carcinoma tissue. Horm Mol Biol Clin Investig. 2012;12:377–90.
Kong SL, Li G, Loh SL, Sung W-K, Liu ET. Cellular reprogramming by the conjoint action of ERa, FOXA1, and GATA3 to a ligand-inducible growth state. Mol Syst Biol. 2011;7:1–14.
Manavathi B. Estrogen receptor coregulators and pioneer factors: the orchestrators of mammary gland cell fate and development. Front Cell Dev Biol. 2014;2:1–13.
Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes Dev. 2008;22:581–6.
Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Dev Biol. 2009;329:227–41.
Lee HJ, Gallego-Ortega D, Ledger A, Schramek D, Joshi P, Szwarc MM, et al. Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development. 2013;140:1397–401.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28:1143–58.
Lim E, Wu D, Pal B, Bouras T, Asselin-Labat M-L, Vaillant F, et al. Transcriptome analyses of mouse and human mammary cell subpopulations reveal multiple conserved genes and pathways. Breast Cancer Res. 2010;12:R21.
Rios AC, Fu NY, Lindeman GJ, Visvader JE. In situ identification of bipotent stem cells in the mammary gland. Nature. 2014;506:322–7.
Chakrabarti R, Wei Y, Romano R-A, DeCoste C, Kang Y, Sinha S. Elf5 regulates mammary gland stem/progenitor cell fate by influencing notch signaling. Stem Cells. 2012;30:1496–508.
Kalyuga M, Gallego-Ortega D, Lee HJ, Roden DL, Cowley MJ, Caldon CE, et al. ELF5 suppresses estrogen sensitivity and underpins the acquisition of antiestrogen resistance in luminal breast cancer. PLoS Biol. 2012;10:e1001461.
Sebastian T, Johnson PF. Stop and go: anti-proliferative and mitogenic functions of the transcription factor C/EBPbeta. Cell Cycle. 2006;5:953–7.
Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, et al. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–28.
Grimm SL, Contreras A, Barcellos-Hoff MH, Rosen JM. Cell cycle defects contribute to a block in hormone-induced mammary gland proliferation in CCAAT/enhancer-binding protein (C/EBPbeta)-null mice. J Biol Chem. 2005;280:36301–9.
Liu Q, Boudot A, Ni J, Hennessey T, Beauparlant SL, Rajabi HN, et al. Cyclin D1 and C/EBP LAP1 operate in a common pathway to promote mammary epithelial cell differentiation. Mol Cell Biol. 2014;34:3168–79.
Frech MS, Torre KM, Robinson GW, Furth PA. Loss of cyclin D1 in concert with deregulated estrogen receptor α expression induces DNA damage response activation and interrupts mammary gland morphogenesis. Oncogene. 2007;27:3186–93.
Grimm SL, Rosen JM. The role of C/EBPbeta in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003;8:191–204.
Liang X-H, Zhao Z-A, Deng W-B, Tian Z, Lei W, Xu X, et al. Estrogen regulates amiloride-binding protein 1 through CCAAT/enhancer-binding protein-β in mouse uterus during embryo implantation and decidualization. Endocrinology. 2010;151:5007–16.
Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein β is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology. 2010;151:3929–40.
Ramathal C, Bagchi IC, Bagchi MK. Lack of CCAAT enhancer binding protein beta in uterine epithelial cells impairs estrogen-induced DNA replication, induces DNA damage response pathways, and promotes apoptosis. Mol Cell Biol. 2010;30:1607–19.
Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–24.
Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, et al. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development. Mol Endocrinol. 2002;16:2675–91.
Kang JH, Tsai-Morris CH, Dufau ML. Complex formation and interactions between transcription factors essential for human prolactin receptor gene transcription. Mol Cell Biol. 2011;31:3208–22.
Goldhar AS, Duan R, Ginsburg E, Vonderhaar BK. Progesterone induces expression of the prolactin receptor gene through cooperative action of Sp1 and C/EBP. Mol Cell Endocrinol. 2011;335:148–57.
Dong J, Tsai-Morris C-H, Dufau ML. A novel estradiol/estrogen receptor alpha-dependent transcriptional mechanism controls expression of the human prolactin receptor. J Biol Chem. 2006;281:18825–36.
Fujimori K, Amano F. Forkhead transcription factor Foxa1 is a novel target gene of C/EBPβ and suppresses the early phase of adipogenesis. Gene. 2011;473:150–6.
Sutinen P, Malinen M, Heikkinen S, Palvimo JJ. SUMOylation modulates the transcriptional activity of androgen receptor in a target gene and pathway selective manner. Nucleic Acids Res. 2014;42:8310–9.
van Bragt MP, Hu X, Xie Y, Li Z. RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells. eLife. 2014;3:e03881.
Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–12.
Owens TW, Rogers RL, Best SA, Ledger A, Mooney AM, Ferguson A, et al. Runx2 is a novel regulator of mammary epithelial cell fate in development and breast cancer. Cancer Res. 2014;74:5277–86.
Chimge N-O, Baniwal SK, Little GH, Chen Y-B, Kahn M, Tripathy D, et al. Regulation of breast cancer metastasis by Runx2 and estrogen signaling: the role of SNAI2. Breast Cancer Res. 2011;13:R127.
Huang B, Qu Z, Ong CW, Tsang Y-HN, Xiao G, Shapiro D, et al. RUNX3 acts as a tumor suppressor in breast cancer by targetingestrogen receptor a. Oncogene. 2011;31:527–34.
Blyth K, Vaillant F, Jenkins A, McDonald L, Pringle MA, Huser C, et al. Runx2 in normal tissues and cancer cells: a developing story. Blood Cell Mol Dis. 2010;45:117–23.
Wang L, Brugge JS, Janes KA. Intersection of FOXO- and RUNX1-mediated gene expression programs in single breast epithelial cells during morphogenesis and tumor progression. Proc Natl Acad Sci. 2011;108:E803–12.
Wall EH, Case LK, Hewitt SC, Nguyen-Vu T, Candelaria NR, Teuscher C, et al. Genetic control of ductal morphology, estrogen-induced ductal growth, and gene expression in female mouse mammary gland. Endocrinology. 2014;155:3025–35.
Douglas NC, Papaioannou VE. The T-box transcription factors TBX2 and TBX3 in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18:143–7.
Arendt LM, St Laurent J, Wronski A, Caballero S, Lyle SR, Naber SP, et al. Human breast progenitor cell numbers are regulated by WNT and TBX3. PLoS ONE. 2014;9:e111442.
Chitilian JM, Thillainadesan G, Manias JL, Chang WY, Walker E, Isovic M, et al. Critical components of the pluripotency network are targets for the p300/CBP interacting protein (p/CIP) in embryonic stem cells. Stem Cells. 2014;32:204–15.
Fan W, Huang X, Chen C, Gray J, Huang T. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. Cancer Res. 2004;64:5132–9.
Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci. 2010;107:21737–42.
Li J, Weinberg MS, Zerbini L, Prince S. The oncogenic TBX3 is a downstream target and mediator of the TGF- 1 signaling pathway. Mol Biol Cell. 2013;24:3569–76.
Seksenyan A, Kadavallore A, Walts AE, de la Torre B, Berel D, Strom SP, et al. TOX3 is expressed in mammary ER+ epithelial cells and regulates ER target genes in luminal breast cancer. BMC Cancer. 2015;15:52.
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4.
Funding Information
This work was supported by grants from the National Health and Medical Research Council of Australia (ID 1008349; ID 1084416) and Cancer Australia (ID 627229), the National Breast Cancer Foundation (ID PS-15-041), a Fellowship Award from the US Department of Defense Breast Cancer Research Program (BCRP; W81XWH-11-1-0592) and a Florey Fellowship from the Royal Adelaide Hospital Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarulli, G.A., Laven-Law, G., Shakya, R. et al. Hormone-Sensing Mammary Epithelial Progenitors: Emerging Identity and Hormonal Regulation. J Mammary Gland Biol Neoplasia 20, 75–91 (2015). https://doi.org/10.1007/s10911-015-9344-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-015-9344-1