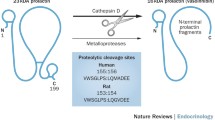
Abstract
There is increasing evidence that prolactin (PRL) and growth hormone (GH) act as growth-promoters of breast tumors. Recent arguments have accumulated to suggest that when they are locally-produced within the mammary tissue, these hormones, acting by an autocrine-paracrine mechanism may have enhanced, or even specific functions compared to endocrine PRL and GH. Classical drugs blocking pituitary hormone production (dopamine and somatostatin analogs) are ineffective on extrapituitary expression of PRL/GH genes, therefore the undesirable effects of these locally-produced hormones remain a target of interest for alternative strategies. This has encouraged the development of competitive PRL and/or GH receptor antagonists, which involve engineered variants of natural receptor ligands (PRL or GH) aimed at blocking receptor activation rather than hormone production in peripheral tissues. This article overviews the rational design of this new class of molecules, their specific molecular features (receptor specificity, biological properties, etc.) and whenever available, the data that have been obtained in cell or animal models of breast cancer.




Similar content being viewed by others
Abbreviations
- PRL:
-
prolactin
- GH:
-
growth hormone
- PRLR:
-
PRL receptor
- GHR:
-
GH receptor
- bp:
-
binding protein
- h:
-
human
- r:
-
rat
- o:
-
ovine
- PEG:
-
polyethylene glycol
References
Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27:485–534.
Welsch CW, Nagasawa H. Prolactin and murine mammary tumorigenesis: a review. Cancer Res. 1977;37:951–63.
Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26:400–22.
Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27.
Vonderhaar BK. Prolactin involvement in breast cancer. Endocr Relat Cancer. 1999;6:389–404.
Ben Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–63.
Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–69.
Molitch ME. Medical management of prolactin-secreting pituitary adenomas. Pituitary. 2002;5:55–65.
Arafah BM, Nasrallah MP. Pituitary tumors: pathophysiology, clinical manifestations and management. Endocr Relat Cancer. 2001;8:287–305.
Fritze D, Queisser W, Schmid H, Kaufmann M, Massner B, Westerhausen M, et al. Prospective randomized trial concerning hyper- and normoprolactinemia and the use of bromoergocryptine in patients with metastatic breast cancer. Onkologie. 1986;9:305–12.
Bonneterre J, Mauriac L, Weber B, Roche H, Fargeot P, Tubiana-Hulin M, et al. Tamoxifen plus bromocriptine versus tamoxifen plus placebo in advanced breast cancer: results of a double blind multicentre clinical trial. Eur J Cancer Clin Oncol. 1988;24:1851–3.
Horti J, Figg WD, Weinberger B, Kohler D, Sartor O. A phase II study of bromocriptine in patients with androgen-independent prostate cancer. Oncol Rep. 1998;5:893–6.
Clevenger CV. Nuclear localization and function of polypeptide ligands and their receptors: a new paradigm for hormone specificity within the mammary gland? Breast Cancer Res. 2003;5:181–7.
Ben Jonathan N, Liby K, McFarland M, Zinger M. Prolactin as an autocrine/paracrine growth factor in human cancer. Trends Endocrinol Metab. 2002;13:245–50.
Llovera M, Touraine P, Kelly PA, Goffin V. Involvement of prolactin in breast cancer: redefining the molecular targets. Exp Gerontol. 2000;35:41–51.
Rose-Hellekant TA, Arendt LM, Schroeder MD, Gilchrist K, Sandgren EP, Schuler LA. Prolactin induces ERalpha-positive and ERalpha-negative mammary cancer in transgenic mice. Oncogene. 2003;22:4664–74.
Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, et al. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–74.
Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–82.
Kindblom J, Dillner K, Sahlin L, Robertson F, Ormandy C, Tornell J, et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology. 2003;144:2269–78.
Manhes C, Kayser C, Bertheau P, Kelder B, Kopchick JJ, Kelly PA, et al. Local over-expression of prolactin in differentiating mouse mammary gland induces functional defects and benign lesions, but no carcinoma. J Endocrinol 2006;190:271–85.
Zinger M, McFarland M, Ben Jonathan N. Prolactin expression and secretion by human breast glandular and adipose tissue explants. J Clin Endocrinol Metab. 2003;88:689–96.
Manfroid I, Van De WC, Baudhuin A, Martial JA, Muller M. EGF stimulates Pit-1 independent transcription of the human prolactin pituitary promoter in human breast cancer SK-BR-3 cells through its proximal AP-1 response element. Mol Cell Endocrinol. 2005;229:127–39.
Baudhuin A, Manfroid I, Van De WC, Martial JA, Muller M. Transcription of the human prolactin gene in mammary cells. Ann N Y Acad Sci. 2002;973:454–8.
Mertani HC, Zhu T, Goh EL, Lee KO, Morel G, Lobie PE. Autocrine human growth hormone (hGH) regulation of human mammary carcinoma cell gene expression. Identification of CHOP as a mediator of hGH stimulated human mammary carcinoma cell survival. J Biol Chem 2001;276:21464–75.
Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, et al. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. 1998;79:202–11.
Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE. Phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc Natl Acad Sci U S A. 2004;101:15166–71.
Kaulsay KK, Zhu T, Bennett W, Lee K, Lobie PE. The effects of autocrine human growth hormone (hGH) on human mammary carcinoma cell behavior are mediated via the hGH receptor. Endocrinology. 2001;142:767–77.
Waters MJ, Barclay JL. Does growth hormone drive breast and other cancers? Endocrinology. 2007;148:4533–5.
Teilum K, Hoch JC, Goffin V, Kinet S, Martial JA, Kragelund BB. Solution structure of human prolactin. J Mol Biol. 2005;351:810–23.
De Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255:306–12.
Horseman ND, Yu-Lee LY. Transcriptional regulation by the helix bundle peptide hormones: growth hormone, prolactin, and hematopoietic cytokines. Endocr Rev. 1994;15:627–49.
Kelly PA, Djiane J, Banville D, Ali S, Edery M, Rozakis M. The growth hormone/prolactin receptor gene family. In: Maclean N, editor. Oxford surveys on eukaryotic genes. London: Oxford University Press; 1991. p. 29–50.
Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, et al. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77.
Wells JA, De Vos AM. Hematopoietic receptor complexes. Annu Rev Biochem. 1996;65:609–34.
Elkins PA, Christinger HW, Sandowski Y, Sakal E, Gertler A, De Vos AM, et al. Ternary complex between placental lactogen and the extracellular domain of the prolactin receptor. Nat Struct Biol. 2000;7:808–15.
Fuh G, Cunningham BC, Fukunaga R, Nagata S, Goeddel DV, Wells JA. Rational design of potent antagonists to the human growth hormone receptor. Science. 1992;256:1677–80.
Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence–function relationships within the expanding family of prolactin, growth hormone, placental lactogen and related proteins in mammals. Endocr Rev. 1996;17:385–410.
Waters MJ, Hoang HN, Fairlie DP, Pelekanos RA, Brown RJ. New insights into growth hormone action. J Mol Endocrinol. 2006;36:1–7.
Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, et al. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12:814–21.
Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and hetero-dimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol. 2006;20:1912–23.
Gadd SL, Clevenger CV. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol. 2006;20:2734–46.
Tan D, Johnson DA, Wu W, Zeng L, Chen YH, Chen WY, et al. Unmodified prolactin (PRL) and S179D PRL-initiated bioluminescence resonance energy transfer between homo- and hetero-pairs of long and short human prolactin receptors in living human cells. Mol Endocrinol. 2005;19:1291–303.
James JR, Oliveira MI, Carmo AM, Iaboni A, Davis SJ. A rigorous experimental framework for detecting protein oligomerization using bioluminescence resonance energy transfer. Nat Methods. 2006;3:1001–6.
Gadd SL, Clevenger CV. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol. 2006;20:2734–46.
Chen WY, Wight DC, Mehta BV, Wagner TE, Kopchick JJ. Glycine 119 of bovine growth hormone is critical for growth-promoting activity. Mol Endocrinol. 1991;5:1845–52.
Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A. 1990;87:5061–5.
Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23:623–46.
Clackson T, Ultsch MH, Wells JA, De Vos AM. Structural and functional analysis of the 1:1 growth hormone:receptor complex reveals the molecular basis for receptor affinity. J Mol Biol. 1998;277:1111–28.
Fuh G, Colosi P, Wood WI, Wells JA. Mechanism-based design of prolactin receptor antagonists. J Biol Chem. 1993;268:5376–81.
Fuh G, Wells JA. Prolactin receptor antagonists that inhibit the growth of breast cancer cell lines. J Biol Chem. 1995;270:13133–7.
Kaulsay KK, Mertani HC, Tornell J, Morel G, Lee KO, Lobie PE. Autocrine stimulation of human mammary carcinoma cell proliferation by human growth hormone. Exp Cell Res. 1999;250:35–50.
Ross RJ, Leung KC, Maamra M, Bennett W, Doyle N, Waters MJ, et al. Binding and functional studies with the growth hormone receptor antagonist, B2036-PEG (pegvisomant), reveal effects of pegylation and evidence that it binds to a receptor dimer. J Clin Endocrinol Metab. 2001;86:1716–23.
Goffin V, Bernichtein S, Carrière O, Bennett WF, Kopchick JJ, Kelly PA. The human growth hormone antagonist B2036 does not interact with the prolactin receptor. Endocrinology. 1999;140:3853–6.
Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, et al. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;278:35988–99.
Bernat B, Pal G, Sun M, Kossiakoff AA. Determination of the energetics governing the regulatory step in growth hormone-induced receptor homodimerization. Proc Natl Acad Sci U S A. 2003;100:952–7.
Walsh ST, Jevitts LM, Sylvester JE, Kossiakoff AA. Site2 binding energetics of the regulatory step of growth hormone-induced receptor homodimerization. Protein Sci. 2003;12:1960–70.
Gent J, Van Den EM, van Kerkhof P, Strous GJ. Dimerization and signal transduction of the growth hormone receptor. Mol Endocrinol. 2003;17:967–75.
Harding PA, Wang X, Okada S, Chen WY, Wan W, Kopchick JJ. Growth hormone (GH) and a GH antagonist promote GH receptor dimerization and internalization. J Biol Chem. 1996;271:6708–12.
Jomain JB, Tallet E, Broutin I, Hoos S, Van Agthoven J, Ducruix A, et al. Structural and thermodynamical bases for the design of pure prolactin receptor antagonists. X-ray structure of Del1–9-G129R-hPRL. J Biol Chem. 2007;282:33118–31.
Bernichtein S, Kinet S, Jeay S, Madern M, Martial JA, Kelly PA, et al. S179D-hPRL, a pseudo-phosphorylated human prolactin analog, is an agonist and not an antagonist. Endocrinology. 2001;142:3950–63.
Kinet S, Bernichtein S, Kelly PA, Martial JA, Goffin V. Biological properties of human prolactin analogs depend not only on global hormone affinity, but also on the relative affinities of both receptor binding sites. J Biol Chem. 1999;274:26033–43.
Goffin V, Struman I, Mainfroid V, Kinet S, Martial JA. Evidence for a second receptor binding site on human prolactin. J Biol Chem. 1994;269:32598–606.
Mode A, Tollet P, Wells T, Carmignac DF, Clark RG, Chen WY, et al. The human growth hormone (hGH) antagonist G120RhGH does not antagonize GH in the rat, but has paradoxical agonist activity, probably via the prolactin receptor. Endocrinology. 1996;137:447–54.
Chen WY, Chen NY, Yun J, Wagner TE, Kopchick JJ. In vitro and in vivo studies of antagonistic effects of human growth hormone analogs. J Biol Chem. 1994;269:15892–7.
Ormandy CJ, Camus A, Barra J, Damotte D, Lucas BK, Buteau H, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–78.
Shen Q, Lantvit DD, Lin Q, Li Y, Christov K, Wang Z, et al. Advanced rat mammary cancers are growth hormone dependent. Endocrinology. 2007;148:4536–44.
Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, et al. Inhibition of estrogen independent mammary carcinogenesis by disruption of growth hormone signaling.. Carcinogenesis. 2006;28:143–50.
Divisova J, Kuiatse I, Lazard Z, Weiss H, Vreeland F, Hadsell DL, et al. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;98:315–27.
Llovera M, Pichard C, Bernichtein S, Jeay S, Touraine P, Kelly PA, et al. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene. 2000;19:4695–705.
Bernichtein S, Jeay S, Vaudry R, Kelly PA, Goffin V. New homologous bioassays for human lactogens show that agonism or antagonism of various analogs is a function of assay sensitivity. Endocrine. 2003;20:177–90.
Ramamoorthy P, Sticca R, Wagner TE, Chen WY. In vitro studies of a prolactin antagonist, hPRL-G129R in human breast cancer cells. Int J Oncol. 2001;18:25–32.
Beck MT, Peirce SK, Chen WY. Regulation of bcl-2 gene expression in human breast cancer cells by prolactin and its antagonist, hPRL-G129R. Oncogene. 2002;21:5047–55.
Peirce SK, Chen WY. Human prolactin and its antagonist, hPRL-G129R, regulate bax and bcl-2 gene expression in human breast cancer cells and transgenic mice. Oncogene. 2004;23:1248–55.
Chen NY, Holle L, Li W, Peirce SK, Beck MT, Chen WY. In vivo studies of the anti-tumor effects of a human prolactin antagonist, hPRL-G129R. Int J Oncol. 2002;20:813–8.
Tomblyn S, Langenheim JF, Jacquemart IC, Holle E, Chen WY. The role of human prolactin and its antagonist, G129R, in mammary gland development and DMBA-initiated tumorigenesis in transgenic mice. Int J Oncol. 2005;27:1381–9.
Bernichtein S, Jomain JB, Kelly PA, Goffin V. The N-terminus of human prolactin modulates its biological properties. Mol Cell Endocrinol. 2003;208:11–21.
Sivaprasad U, Canfield JM, Brooks CL. Mechanism for ordered receptor binding by human prolactin. Biochemistry. 2004;43:13755–65.
Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–36.
Ma FY, Grattan DR, Goffin V, Bunn SJ. Prolactin-regulated tyrosine hydroxylase activity and messenger ribonucleic acid expression in mediobasal hypothalamic cultures: the differential role of specific protein kinases. Endocrinology. 2005;146:93–102.
Dagvadorj A, collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–101.
Jordan VC, Murphy CS. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990;11:578–610.
Fischer OM, Streit S, Hart S, Ullrich A. Beyond Herceptin and Gleevec. Curr Opin Chem Biol. 2003;7:490–5.
Chen WY, Ramamoorthy P, Chen N, Sticca R, Wagner TE. A human prolactin antagonist, hPRL-G129R, inhibits breast cancer cell proliferation through induction of apoptosis. Clin Cancer Res. 1999;5:3583–93.
Scotti ML, Langenheim JF, Tomblyn S, Springs AE, Chen WY. Additive effects of a prolactin receptor antagonist, G129R, and herceptin on inhibition of HER2-overexpressing breast cancer cells. Breast Cancer Res Treat. 2008. doi 10.1007/s10549-007-9789-z.
Yamauchi T, Yamauchi N, Ueki K, Sugiyama T, Waki H, Miki H, et al. Constitutive tyrosine phosphorylation of ErbB-2 via Jak2 by autocrine secretion of prolactin in human breast cancer. J Biol Chem. 2000;275:33937–44.
Zhang G, Li W, Holle L, Chen N, Chen WY. A novel design of targeted endocrine and cytokine therapy for breast cancer. Clin Cancer Res. 2002;8:1196–205.
Garcia-Tunon I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. Interleukin-2 and its receptor complex (alpha, beta and gamma chains) in in situ and infiltrative human breast cancer: an immunohistochemical comparative study. Breast Cancer Res. 2004;6:R1–7.
Beck MT, Chen NY, Franek KJ, Chen WY. Prolactin antagonist–endostatin fusion protein as a targeted dual-functional therapeutic agent for breast cancer. Cancer Res. 2003;63:3598–604.
Tomblyn S, Springs AE, Langenheim JF, Chen WY. A multifaceted, targeted combination therapy using three prolactin receptor antagonist based fusion proteins which significantly inhibits tumor recurrence in HER2/neu mice. 89th Meeting of the Endocrine Society Toronto, Canada (june 2–5)[OR-43-1]. 2007, Abstract.
Goffin V, Touraine P, Culler MD, Kelly PA. Drug Insight: prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat Clin Pract Endocrinol Metab. 2006;2:571–81.
van den Eijnden MJ, Strous GJ. Autocrine growth hormone: effects on growth hormone receptor trafficking and signaling. Mol Endocrinol. 2007;21:2832–46.
Oakes SR, Robertson FG, Kench JG, Gardiner-Garden M, Wand MP, Green JE, et al. Loss of mammary epithelial prolactin receptor delays tumor formation by reducing cell proliferation in low-grade preinvasive lesions. Oncogene. 2007;26:543–53.
Kelly PA, Bachelot A, Kedzia C, Hennighausen L, Ormandy CJ, Kopchick JJ, et al. The role of prolactin and growth hormone in mammary gland development. Mol Cell Endocrinol. 2002;197:127–31.
Nouhi Z, Chughtai N, Hartley S, Cocolakis E, Lebrun JJ, Ali S. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66:1824–32.
Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746–60.
Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–60.
Maus MV, Reilly SC, Clevenger CV. Prolactin as a chemoattractant for human breast carcinoma. Endocrinology. 1999;140:5447–50.
Gourdou I, Paly J, Hue-Beauvais C, Pessemesse L, Clark J, Djiane J. Expression by transgenesis of a constitutively active mutant form of the prolactin receptor induces premature abnormal development of the mouse mammary gland and lactation failure. Biol Reprod. 2004;70:718–28.
Manhes C, Kayser C, Bertheau P, Kelder B, Kopchick JJ, Kelly PA, et al. Local over-expression of prolactin in differentiating mouse mammary gland induces functional defects and benign lesions, but no carcinoma. J Endocrinol. 2006;190:271–85.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tallet, E., Rouet, V., Jomain, JB. et al. Rational Design of Competitive Prolactin/Growth Hormone Receptor Antagonists. J Mammary Gland Biol Neoplasia 13, 105–117 (2008). https://doi.org/10.1007/s10911-008-9066-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-008-9066-8