Abstract
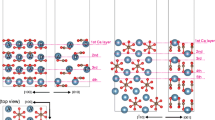
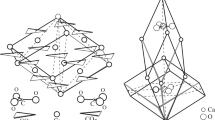
We have investigated the presence of foreign ions into the bulk structure and the external surfaces of aragonite using periodic ab-initio methods. Four cations isovalent to Ca2+ were studied: Mg2+, Sr2+, Ba2+ and Zn2+. The calculations were performed at structures (bulk, surface) that contain four and eight CaCO3 units. Our results, at the Hartree-Fock level, show that the incorporation of those ions into aragonite depends strongly on their size. Mg2+ and Zn2+, due to their smaller size, can substitute Ca2+ ions in the crystal lattice while the incorporation of Sr2+ and Ba2+ into aragonite is energetically less favoured. Examination of the [011], [110] and [001] surfaces of aragonite revealed that the surface incorporation reduces the energetic cost for the larger ions. These systems provide challenging examples for most shape analysis methods applied in Mathematical Chemistry.
Similar content being viewed by others
References
Cowan J.C., Weintritt D.J.: Water Formed Scale Deposits, p. 112. Gulf, Houston, TX (1976)
Aizemberg J., Black A.J., Whitesides G.: Nature 398, 495 (1999)
Han Y.J., Aizemberg J.: J. Am. Chem. Soc. 125, 4032 (2003)
Lakshminarayanan R., Valiyaveettil S., Luan Loy G. Cryst. Growth Des. 3, 953 (2003)
Zhang Y., Dawe R.A.: Chem. Geol. 163, 129 (2000)
D’Souza S.M., Alexander C., Carr S.W., Waller A.M., Whithcombe M.J., Vulfon E.N.: Nature 23, 513 (1999)
Dalas E., Klepetsanis P., Koutsoukos P.G.: Langmuir 15, 8322 (1999)
Rautaray D., Sanyal A., Bharde A., Ahmad A., Sastry M.: Cryst. Growth Des. 5, 399 (2005)
Clarkson J.R., Price T.J., Adams C.J.: J. Chem. Soc. Faraday Trans. 88, 243 (1992)
N-Laslo V., Brecevic L.: J. Chem. Soc. Faraday Trans. 94, 2005 (1998)
Fujita Y., Redden G.D., Ingram J.C., Cortez M.M., Ferris F.G., Smith R.W.: Geochimica et Cosmochimica Acta 68, 3261 (2004)
Hwang S., Blanco M., Goddard W.A. III: J. Phys. Chem. B 105, 10746 (2001)
Nygren M.A., Gay D.H., Richard C., Catlow A., Wilson M.P., Rohl A.L.: J. Chem. Soc. Faraday Trans. 94, 3685 (1998)
Catti M., Pavese A., Apra E., Roetti C.: Phys. Chem. Miner. 20, 104 (1993)
Weber H.J.: Acta Crystallogr. A 44, 320 (1988)
Rohl A.L., Wright K., Gale J.D.: Am. Mineral. 88, 921 (2003)
Pohl D., Rath R.: Acta Crystallogr. A 35, 694 (1979)
de Leeuw N.H., Parker S.C.: J. Phys. Chem. B 102, 2914 (1998)
Sabbides T., Giannimaras E.K., Koutsoukos P.G.: Environ. Technol. 13, 73 (1992)
Rimstidt J.D., Balog A., Webb J.: Geochim. Cosmochim. Acta 62, 1851 (1998)
Giles R., Manne S., Mann S., Morse D.E., Stucky G.D., Hansma P.K.: Biol. Bull. 188, 8 (1995)
Nancollas G.H., Sawada M.M.: J. Petroleum Techn. 34, 76 (1982)
Santillan J., Williams Q.: Phys. Earth Planet. In. 143-144, 291 (2004)
Lea D.W., Pak D.K., Spero H.J.: Science 289, 1719 (2000)
Lea D.W., Shen G.T., Boyle E.A.: Nature 340, 373 (1989)
Adkins J.F., Cheng H., Boyle E.A., Druffel E.R.M., Edwards R.L.: Science 280, 725 (1998)
Boyle E.A.: Science 249, 863 (1990)
Schettler G., Pearce N.J.G.: Hydrobiologia 317, 1 (1996)
Price G.D., Pearce N.J.G.: Mar. Poll. Bull. 34, 1025 (1997)
G.T. Shen, C.L. Sanford (1990) in Global Ecological Consequences of the 1982-83 El Nino-Southern Oscillation, ed. by P.W. Glynn (Elsevier.4097 Barium and strontium in protoconch and statolith), p. 255
Tudhope A.W., Chilcott C.P., McCulloch M.T., Cook E.R., Chappell J., Ellam R., Lea D.W., Lough J.M., Shimmield G.B.: Science 291, 1511 (2001)
Wells B.K., Bath G.E., Thorrold S.E., Jones C.M.: Can. J. Fish. Aquat. Sci. 57, 2122 (2000)
Menadakis M., Maroulis G., Koutsoukos P.G.: Comput. Mater. Sci. 38, 522 (2007)
Saunders V.R., Dovesi R., Roetti C., Causa M., Harrison N.M., Orlando R., Zicovich-Wilson C.M.: CRYSTAL98 User’s Manual. Universita di Torino, Torino (1999)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Menadakis, M., Maroulis, G. & Koutsoukos, P.G. Incorporation of Mg2+, Sr2+, Ba2+ and Zn2+ into aragonite and comparison with calcite. J Math Chem 46, 484–491 (2009). https://doi.org/10.1007/s10910-008-9490-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10910-008-9490-4